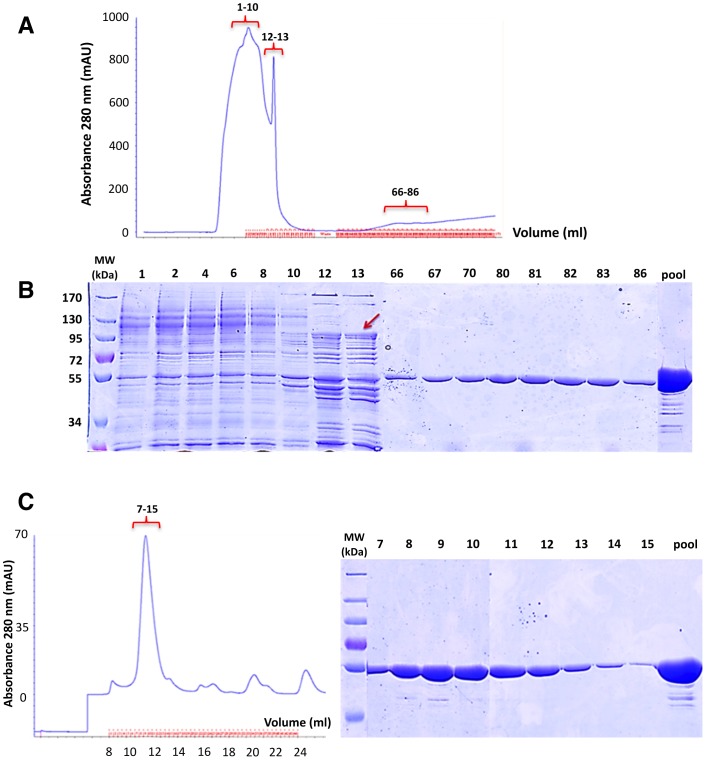
Figure 6. Strategy to eliminate the AcrB contamination.
A, chromatogram of DDM-solubilized BmrA eluted from a Ni2+-High Trap chelating column. The absorbance of the protein was monitored at 280 nm. The column was equilibrated and washed with 50 mM imidazole. B, purification of BmrA was analysed by 10% SDS-PAGE. Fractions 1–10: Proteins loaded on Ni2+-High Trap chelating column, 12–13: BmrA washed with 50 mM imidazole, 66–86: BmrA eluted by linear imidazole gradient from 50 to 250 mM. The Red arrow indicates the position of AcrB. C, left panel, size exclusion chromatography of BmrA loaded onto a Superdex 200 10/300 GL column. Right panel, the fractions 7–15 collected from the size exclusion chromatography were resolved on a 10% SDS-PAGE.