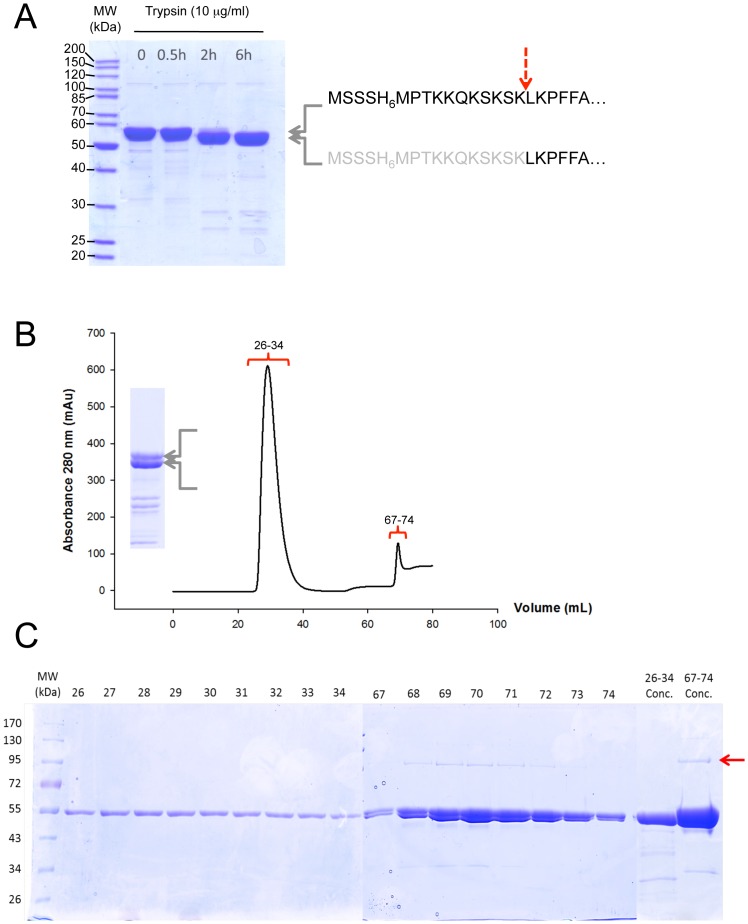
Figure 7. The cleavage of the His6-tag at the N-terminus of BmrA allows the elimination of AcrB by a second Ni2+–NTA chromatographic step.
A, The E504Q BmrA mutant was incubated with 2 mM ATP and MgCl2 during 30 min 23°C, and trypsin (10 µg/mL) was added. At the time indicated, an aliquot was withdrawn, mixed with the SDS-PAGE loading buffer and kept on ice before being submitted to electrophoresis. After 6 h of incubation with trypsin, the ‘lower’ band migrating just below the full-length BmrA was identified by Edman sequencing and shown to correspond to BmrA lacking its N-terminal extremity and starting at Leu(12)-Lys(13)-Pro(14)-Phe(15)-Phe(16)… Therefore the main cut with trypsin occurred between Lys(11) and Leu(12) and is indicated by a red dashed arrow. B, After solubilization with DDM of membrane containing overexpressed E504Q BmrA mutant, extracted and non-extracted materials were separated by an ultracentrifugation and the supernatant was loaded onto a Ni2+ high trap chelating column followed by a PD-10 desalting column. The recovered E504Q BmrA mutant was incubated with 2 mM ATP and MgCl2 during 30 min and was submitted to trypsin digestion as in A. The incubation time was chosen to clearly see both the full-length, uncut protein, and BmrA with its N-terminal being cleaved (inset). The mixture was then submitted to a second Ni2+-High Trap chelating column as before and the chromatogram of the protein eluted from the column and monitored at 280 nm is shown. The first peak, fractions 26–34, corresponded to the unbound proteins and the second peak, fractions 67–74, to the proteins eluted with 250 mM imidazole. C, the different fractions obtained in B were analyzed by 10% SDS-PAGE. The two last lanes correspond to fractions 26–34 and 83–90 which were pooled separately and concentrated on a centricon (MWCO 50 kDa) before being submitted to electrophoresis. The Red arrow indicates the position of AcrB.