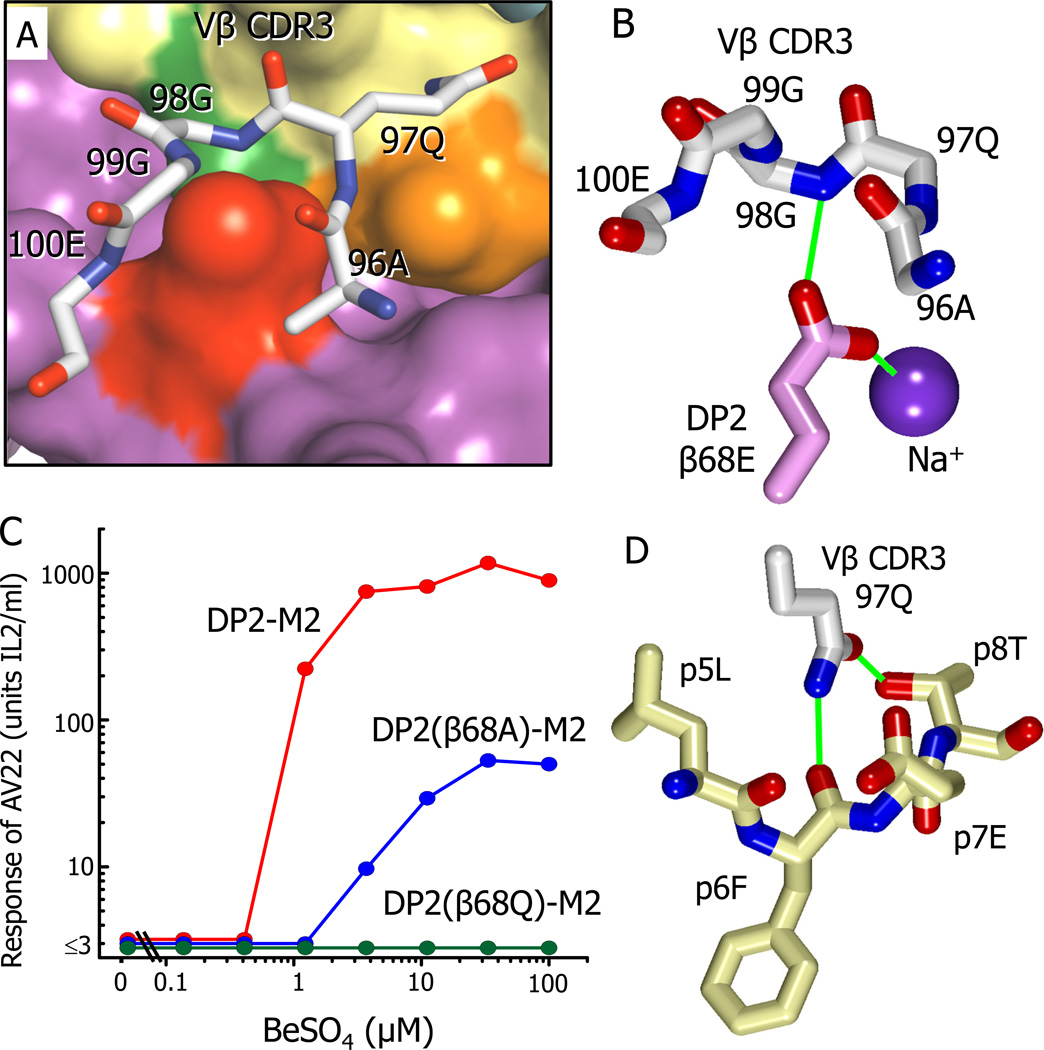
Figure 5. Interaction of AV22 VβCDR3 with the surface of DP2-M2 above the Be2+ binding site.
(A) A wireframe representation is shown of the backbone of the AV22 TCR VβCDR3 loop and the side chain of Vβ97Q interacting with the surface of DP2-M2.
(B) The orientation of the side chain carboxylate of DP2 β68E between the VβCDR3 loop of the AV22 TCR and the internal Na cation is shown. Potential H-bond/salt bridges are shown as green lines.
(C) The response of AV22 without or with various concentrations of BeSO4 presented by the wild-type DP2-M2 complex or the complex mutated at β68E to glutamine or alanine. The experiment was repeated with similar results.
(D) Interactions of the carboxamide of Vβ CDR3 97Q with the M2 peptide are shown. Potential H-bonds are shown as green lines.
See also Figure S2 and Table S2.