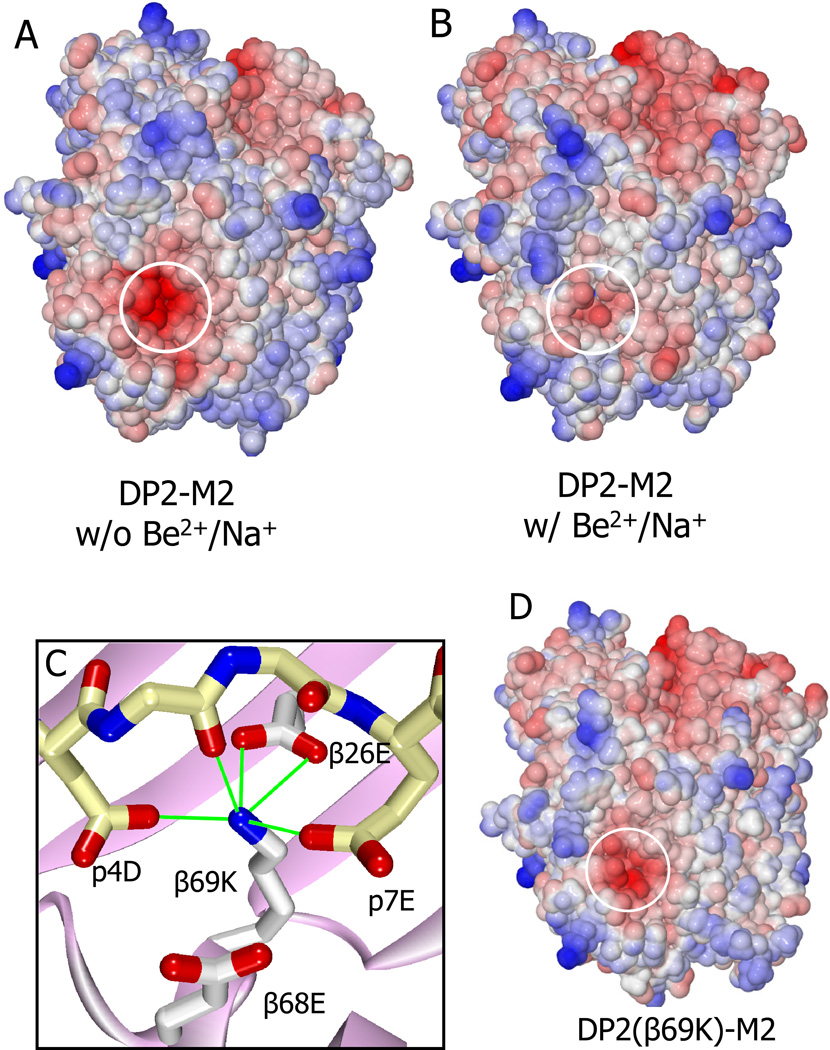
Figure 6. Specific changes in the electrostatic surface potential of the DP2-M2 complex caused by binding Be2+/Na+.
(A and B) Water accessible surface of the DP-M2 complex in the absence (A) or presence (B) of Be2+ and Na+, colored by the electrostatic surface potential (red, negative; blue, positive). Area overlaying the acidic pocket is circled.
(C) The interaction of the β69K lysine ε-amino group in the DP2(β69K)-M2 mutant structure with amino acids in the acidic pocket is shown. Potential H-bond/salt bridges are shown as green lines.
(D) The electrostatic surface potential of the DP2(β69K)-M2 mutant complex is shown as in (A).
See also Table S1 and Table S2.