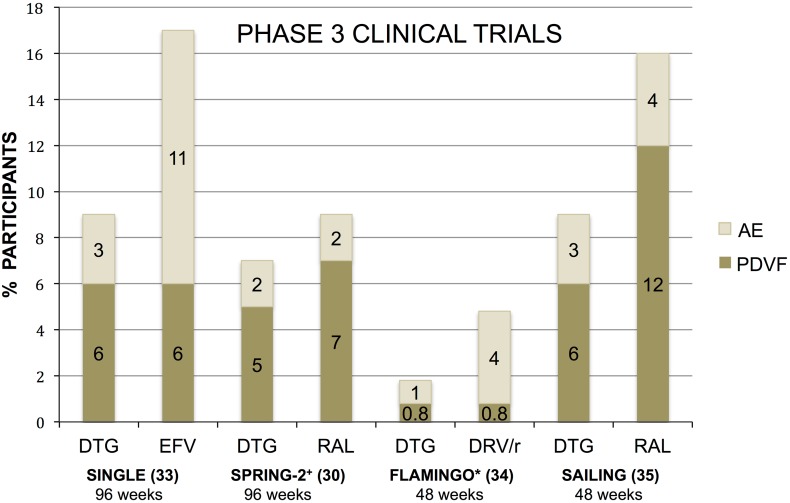
Fig. 2.
Phase 3 clinical trials of DTG and comparator antiretroviral therapy evaluating PDVF criteria versus discontinuation due to adverse events. PDVF defined by study endpoint (>50 copies/mL) including those who never suppressed or those who rebounded; *FLAMINGO study endpoint (>200 copies/mL); +SPRING-2 study endpoint (>50 copies/mL × 2 from week 24–48; then up to 200 copies/mL after week 48). DRV/r darunavir/ritonavir, DTG dolutegravir, EFV efavirenz, PDVF protocol-derived virologic failure, RAL raltegravir