Abstract
About 15% of heterosexual couples in the USA suffer from infertility issues; male infertility accounts for ∼50% of all infertility cases and roughly 50% of male infertility is idiopathic. Increased levels of plasma cholesterol affect spermatogenesis and male fertility negatively, but by unclear mechanisms. Clearly, spermatogenesis occurs in immune-privileged seminiferous tubules that are protected by the blood-testis barrier (BTB), and BTB disruption results in sperm damage and male infertility. Accordingly, using rabbits fed a 2% cholesterol-enriched diet for 2, 4, and 6 wk to raise levels of plasma cholesterol, we tested the hypothesis that elevated levels of plasma cholesterol disrupt the BTB functionally and biochemically. The cholesterol-enriched diet increased lipid deposition dramatically and time-dependently in the seminiferous tubules and disrupted the BTB as evidenced by increased IgG staining within the seminiferous tubules. Total protein levels of the tight-junction proteins ZO-1 and occludin were increased in the seminiferous tubules of rabbits fed the cholesterol-enriched diet, and the distribution patterns of tight-junction proteins were markedly affected, including an increased accumulation of tight-junction proteins in endosomes. Disruption of the integrity of the BTB due to increased plasma levels of cholesterol might play a role in male infertility.
Keywords: cholesterol, male infertility, blood-testis barrier, tight junctions
male infertility contributes to roughly 50% of all infertility cases, and ∼15% of heterosexual couples in the USA experience fertility issues (7, 16). Although varicoceles, obstructions, ejaculatory dysfunction, infections, and hormonal deficiencies are known causes of male infertility, a significant proportion (40–50%) of male infertility is idiopathic (7). Elevated levels of plasma cholesterol in humans can decrease semen quality and contribute to male infertility (10, 14, 17), while high levels in animals can decrease sperm concentration, impair sperm motility, reduce length of sperm midpiece, and lower rates of in vitro fertilization (1, 20, 22, 31). In addition, increased cholesterol accumulation in Sertoli cells reduces testicular function and compromises fertility (15, 18, 21). Thus, it appears clear from human and animal studies that increased plasma cholesterol impairs spermatogenesis and affects male fertility.
Spermatogenesis occurs in seminiferous tubules, an immune-privileged environment that is protected by the blood-testis barrier (BTB). The BTB is a barrier that consists of tight-junction complexes between adjacent Sertoli cells and that divides the seminiferous epithelium into basal and adluminal compartments. The BTB limits the entry of toxins, large hydrophilic molecules, and immune cells into seminiferous tubules, thus creating a unique nurturing environment for developing meiotic and maturing postmeiotic germ cells (5, 27). The importance of the BTB in spermatogenesis is highlighted by findings that BTB dysfunction leads to sperm damage and male infertility (3, 5, 6, 8, 23).
The BTB is a very dynamic structure. During spermatogenesis the BTB rapidly undergoes a complicated and coordinated temporal/spatial disassembly and reassembly of tight junctions (30, 32), most likely due to a dynamic regulation of tight-junction protein trafficking (24, 25, 28–30, 32). Therefore, pathological disturbances in endocytosis and/or recycling of tight-junction proteins could lead to BTB disruption.
Cholesterol homeostasis in Sertoli cells is tightly controlled by a combination of de novo synthesis, cholesterol uptake, and cholesterol efflux (11, 13, 15, 18, 21, 26). And, altered Sertoli cell cholesterol homeostasis impairs testicular function and fertility (15, 18, 21). Because Sertoli cells take up extracellular cholesterol through receptor-mediated endocytosis (9), we hypothesized that elevated levels of plasma cholesterol would increase cholesterol accumulation, promote endocytic accumulation of tight-junction proteins in Sertoli cells, and disrupt BTB integrity. Our findings that rabbits fed a cholesterol-enriched diet exhibit disrupted BTB, cholesterol accumulation within Sertoli cells, and accumulation of tight-junction proteins in endocytic compartments suggest that elevated plasma cholesterol levels might thereby contribute to male infertility.
MATERIALS AND METHODS
Rabbits.
New Zealand white male rabbits (1.5 to 2 yr old) weighing 3–4 kg were fed either normal chow or normal chow supplemented with 2% cholesterol for 2, 4, or 6 wk (n = 4). At necropsy, animals were perfused with Dulbecco's phosphate-buffered saline, and testes were dissected, frozen on a liquid nitrogen cooled surface, and stored at −80°C until taken for experimentation. The animal protocol was approved by the University of North Dakota Animal Care and Use Committee, and adhered to the Guide for the Care and Use of Laboratory Animals (NIH publication no. 80-23).
Cholesterol measurement.
Total serum levels of total cholesterol were measured in blood collected from rabbit ear veins. Lipid levels were measured by standard techniques with an Olympus AU640 clinical analyzer.
Oil red O staining.
Testes were sectioned (thickness 14 μm) using a cryostat (Micron) and were fixed with 10% formalin for 10 min, washed with H2O, and incubated with 60% isopropanol for 5 min. Once dried, sections were stained with Oil red O (Sigma) for 10 min and washed with H2O. Images were acquired using a Leica microscope.
Immunostaining.
Cryostat sections (as described above) were stained for target proteins using antibodies against EEA1 (Santa Cruz Biotechnology), rabbit IgG (Invitrogen), ZO-1 (Invitrogen), and occludin (Invitrogen). Double fluorescence staining was used to determine subcellular codistribution of tight-junction proteins with endosomes and with endogenous IgG. Controls for specificity included staining with an isotype-matched irrelevant antibody as a negative control, staining with primary antibodies without fluorescence-conjugated secondary antibodies (background controls), and staining with only secondary antibodies; these controls eliminated autofluorescence in each channel and bleed-through (crossover) between channels.
Immunoblotting.
Total cell lysates and crude endolysosome fractions were prepared using a endolysosome isolation kit (Sigma). Testes (0.5 g wet wt) were ground into powder and homogenized in 4 volumes of 1× extraction buffer. Homogenates were centrifuged at 1,000 g for 10 min at 4°C. Supernatants (total cell lysate) were collected and then centrifuged at 20,000 g for 20 min at 4°C. Pellets were resuspended in 0.5 ml of 1× extraction buffer, and this fraction contained a mixture of light mitochondria, endosomes, lysosomes, peroxisomes, and endoplasmic reticulum. Protein concentration was determined by Bradford assay. Equal amounts of protein (10 μg) were resolved by SDS-PAGE under reducing conditions, transferred to PVDF membranes, and subjected to immunoblotting with antibodies against rabbit IgG, ZO-1, and occludin. β-Actin (Abcam) was used as a control. Blots were probed with secondary antibodies conjugated with horseradish peroxidase for 1 h at room temperature, reacted with luminal reagent, exposed, visualized, and analyzed by LabWorks 4.5 software on a UVP Bioimaging System (Upland). Quantification was performed by densitometry, and the results were analyzed and normalized.
Statistical analysis.
All data were expressed as means ± SE. Statistical significance was analyzed with one-way ANOVA plus a Tukey post hoc test. P < 0.05 was considered to be statistically significant.
RESULTS
Cholesterol-enriched diet promoted cholesterol accumulation in seminiferous tubules.
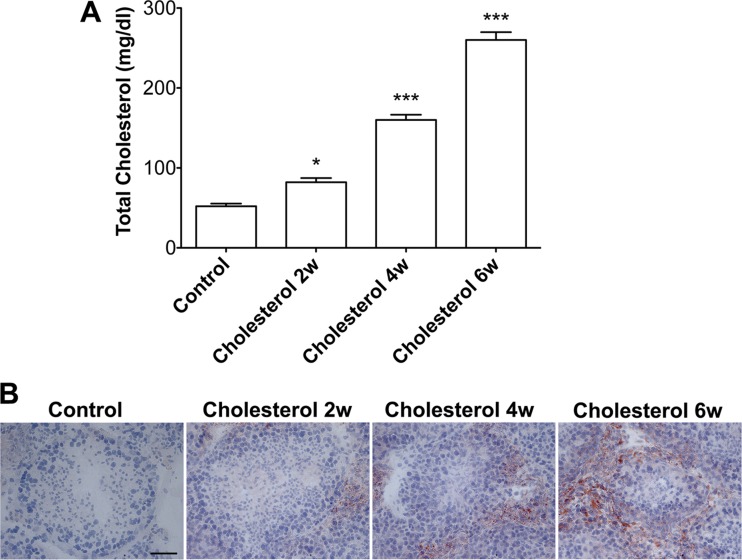
Total plasma levels of cholesterol were markedly increased in rabbits ingesting for 2, 4, and 6 wk a diet supplemented with 2% cholesterol (Fig. 1A). Because Sertoli cells take up extracellular cholesterol through receptor-mediated endocytosis (9), we first determined the effects of cholesterol-enriched diet on cholesterol accumulation in seminiferous tubules. Using an Oil red O staining method, which stains cholesterol and triglycerides, we found that the cholesterol-enriched diet increased lipid droplet accumulation significantly and time-dependently in seminiferous tubules (Fig. 1B).
Fig. 1.
Cholesterol-enriched diet promoted lipid accumulation in seminiferous tubules. A: total plasma levels of cholesterol were markedly increased following feeding rabbits a diet supplemented with 2% cholesterol for 2, 4, and 6 wk (n = 4, *P < 0.05). B: Oil red O staining demonstrated little to no lipid accumulation along or within the seminiferous tubules in testes from control rabbits, but increasing amounts of lipid accumulation were observed in testes from rabbits fed the cholesterol-enriched diet for 2, 4 and 6 wk. Scale bar, 50 μm.
Cholesterol-enriched diet disrupted the BTB.
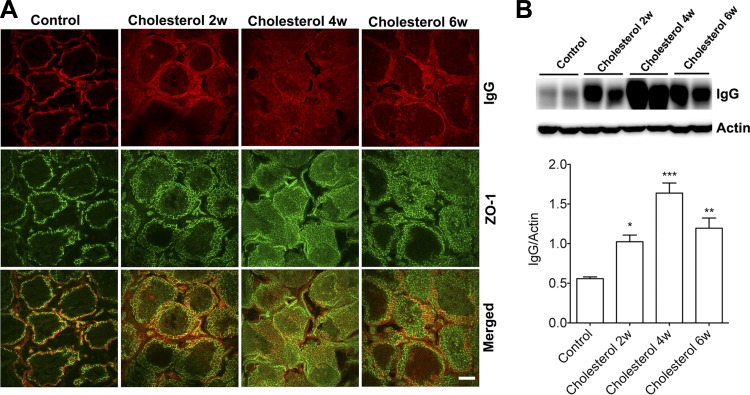
We determined next whether rabbits fed the cholesterol-enriched diet exhibited functional and morphological evidence of BTB dysfunction. BTB integrity was examined using a double fluorescence staining method for endogenous IgG as a marker of BTB leakage and ZO-1 as a marker of tight-junction proteins integral to the BTB. In testes from control rabbits, ZO-1 was localized at the basal regions of the seminiferous tubule, and IgG staining was excluded from the seminiferous tubule (Fig. 2A), observations indicating that the BTB was functionally intact. However, the staining pattern of ZO-1 and IgG changed dramatically in testes from rabbits fed the cholesterol-enriched diet for 2, 4, and 6 wk; more ZO-1 was present in the adluminal region of the seminiferous tubule, and increasingly larger amounts of IgG staining were present within the seminiferous tubule (Fig. 2A). Consistent with leakage of IgG into seminiferous tubules as shown by immunostaining, we demonstrated that the cholesterol-enriched diet dramatically increased protein levels of IgG in testes (Fig. 2B).
Fig. 2.
Cholesterol-enriched diet increased IgG leakage into seminiferous tubules, A: in testes from control rabbits, ZO-1 staining (green) of tight-junction proteins was restricted to the basal regions of the Sertoli cells, and there was no positive IgG staining inside the seminiferous tubules. However, staining patterns of ZO-1 and IgG changed dramatically in testes from rabbits fed a cholesterol-enriched diet for 2, 4, and 6 wk; more ZO-1 was present in the adluminal region of seminiferous tubules and increasingly larger amounts of IgG staining were present within seminiferous tubules. Scale bar, 100 μm. B: cholesterol-enriched diet dramatically increased protein levels of IgG in testes (n = 4, *P < 0.05).
Cholesterol-enriched diet increased accumulation of tight-junction proteins in endocytic compartments.
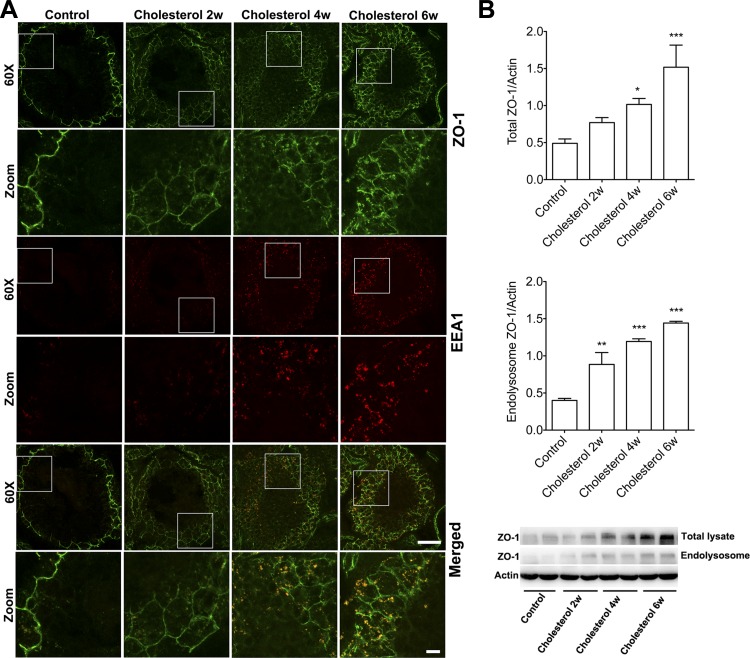
Endocytic trafficking of tight-junction proteins participates in the dynamic regulation of the BTB (24, 28–30, 32). Because elevated levels of plasma cholesterol promote cholesterol accumulation in seminiferous tubules, it might thereby affect endocytic trafficking of tight-junction proteins. Accordingly, we next determined the effects of cholesterol-enriched diet on tight-junction protein internalization using immunostaining and immunoblotting methods. We demonstrated that the cholesterol-enriched diet time-dependently and statistically significantly altered protein levels and distribution patterns of ZO-1 (Fig. 3). In control testes, ZO-1 was distributed only at the base of seminiferous tubules, and very little positive staining for endosomes (EEA1) was present. However, after 2 wk on the cholesterol-enriched diet, ZO-1 staining became apparent in the adluminal side of seminiferous tubules. In addition, the cholesterol-enriched diet markedly increased the numbers of EEA-1-positive endosomes. The effects of the cholesterol diet on ZO-1 and endosomes were increasingly apparent as the rabbits were maintained on the diet for 4 and 6 wk. Importantly, we demonstrated that the cholesterol-enriched diet time-dependently increased the codistribution of ZO-1 with endosomes (Fig. 3A). Consistent with our findings from immunostaining studies, we demonstrated that the cholesterol-enriched diet not only time-dependently increased total protein levels of ZO-1 but also significantly increased protein levels of ZO-1 in crude endolysosome fractions (Fig. 3B).
Fig. 3.
Cholesterol-enriched diet increased ZO-1 accumulation in endocytic compartments. A: in testes from control rabbits, ZO-1 staining (green) was restricted to the basal regions of Sertoli cells, and there was minimal EEA1 positive staining for endosomes (red). Feeding rabbits a cholesterol-enriched diet for 2, 4, and 6 wk dramatically changed the staining patterns of ZO-1 and EEA1; increasing amounts of ZO-1 positive staining were apparent at the adluminal side of seminiferous tubules, and the numbers and sizes of EEA-1-positive endosomes were increased. Importantly, the cholesterol-enriched diet time-dependently increased the codistribution of ZO-1 with EEA-1-positive endosomes. Under ×60 magnification (scale bar, 50 μm;in zoomed-in areas, scale bar, 10 μM). B: cholesterol-enriched diet not only time-dependently increased total protein levels of ZO-1 in testes but also significantly increased protein levels of ZO-1 in endolysosome fractions (n = 4, *P < 0.05).
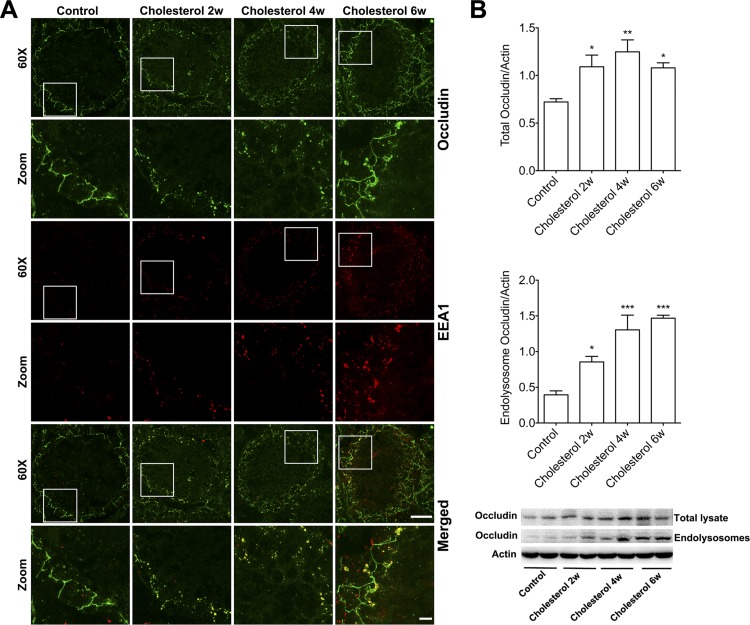
To further confirm our findings, we determined the effects of cholesterol-enriched diet on internalization of occludin, an integral membrane tight-junction protein. Similarly to ZO-1, we demonstrated that cholesterol-enriched diet dramatically altered protein levels and distribution patterns of occludin in a time-dependent manner. In control testes, occludin was distributed only at the base of seminiferous tubules, and very little positive staining for endosomes (EEA1) was present. However, the cholesterol-enriched diet dramatically altered the distribution of occludin immunostaining, with positive punctate staining becoming apparent in the adluminal side of the seminiferous tubules and this punctate staining colocalized with the endosome marker EEA1 (Fig. 4A). For rabbits fed the cholesterol-enriched diet for 2 or 4 wk, the distribution pattern of occludin immunostaining changed dramatically from a linear and barrier-like pattern in controls to a nonlinear and punctate pattern; as in controls, the punctate staining colocalized with EEA1-positive endosomes. For rabbits fed the cholesterol-enriched diet for 6 wk, the punctate staining pattern of occludin persisted, but the linear and barrier-like pattern of occludin reappeared. Consistent with our immunostaining findings, we demonstrated that a cholesterol-enriched diet not only time-dependently increased total protein levels of occludin but also significantly increased protein levels of occludin in crude endolysosome fractions (Fig. 4B).
Fig. 4.
Cholesterol-enriched diet increased occludin accumulation in endocytic compartments. A: in testes from control rabbits, linear and barrier-like occludin staining (green) was restricted to the basal regions of Sertoli cells, and there was minimal punctate staining of occludin that colocalized with EEA1-positive endosomes (red). After 2 and 4 wk on a the cholesterol-enriched diet, the distribution of occludin staining changed dramatically from a linear and barrier-like pattern in controls to a nonlinear and punctate pattern; the punctate staining colocalized with EEA1-positive endosomes. After 6 wk on the cholesterol-enriched diet, the punctate staining for occludin persisted, but the linear and barrier-like pattern of occludin reappeared. Under ×60 magnification (scale bar, 50 μm; in zoomed areas scale bar, 10 μM). B: cholesterol-enriched diet not only time-dependently increased total protein levels of occludin in testes but also significantly increased protein levels of occludin in endolysosome fractions (n = 4, *P < 0.05).
DISCUSSION
The present study tested our hypothesis that elevated levels of plasma cholesterol, as induced by feeding rabbits a diet enriched in cholesterol, would disrupt BTB integrity and disturb expression levels of tight-junction proteins. Rabbits were used for these studies because they are an excellent model for reproductive system research (12) and they exhibit a functional BTB. Rabbits have the additional advantage of being a well-used model for hypercholesterolemia and its pathological consequences including decreased sperm concentration, impaired sperm motility, reduced length of sperm midpiece, and lowers rate of in vitro fertilization (20, 31).
The BTB consists of tight-junction complexes between adjacent Sertoli cells near the base of the seminiferous epithelium (2) in seminiferous tubules where spermatogenesis occurs. Seminiferous tubules are immune privileged, in part because the BTB limits the entry of toxins, large hydrophilic molecules, and immune cells, thus creating a unique nurturing environment for developing germ cells (5, 27). The importance of the BTB in reproductive health is highlighted clearly by findings that BTB dysfunction leads to sperm damage and male infertility (3, 5, 6, 8, 23). At the molecular level, the BTB forms a complex network of tight-junction proteins that are segregated into three major classes: integral membrane proteins, peripheral adaptors and their associated signaling molecules, and cytoskeletal proteins. The cytoplasmic domains of the integral membrane proteins are linked to the actin cytoskeletal network via adaptor proteins. Three types of transmembrane tight-junction proteins exist: junctional adhesion molecules, occludin, and claudins. The major adaptor proteins that connect transmembrane tight-junction proteins to actin cytoskeleton are zonula occludens (ZO-1) (2, 27).
The BTB is a very dynamic structure (2) and undergoes cycles of “opening” and “closing” to accommodate migration of spermatocytes from basal to adluminal compartments. BTB integrity must be maintained for developing meiotic and maturing postmeiotic germ cells (4, 19), and disruption of the BTB leads to sperm damage and male infertility (5). Mechanisms underlying this dynamic reconstruction of tight junctions during spermatogenesis include the relatively new concept of endocytic trafficking of tight-junction proteins (30, 32), and disturbances in endocytosis and/or recycling of tight-junction proteins could play critical roles in disruption of BTB integrity under pathological conditions.
Sertoli cells, which form the BTB, are capable of taking up extracellular cholesterol through receptor-mediated endocytosis (9). Therefore, receptor-mediated endocytosis of cholesterol in Sertoli cells would be enhanced under conditions when plasma cholesterol is high, and this leads to cholesterol accumulation in Sertoli cells. Indeed, in rabbits fed a cholesterol-enriched diet we observed a dramatic increase in cholesterol and triglyceride deposition in seminiferous tubules. Here, we examined BTB integrity using a double fluorescence staining method for endogenous IgG as a marker of BTB leakage and ZO-1 as a marker of tight-junction proteins integral to the BTB. We found that the cholesterol-enriched diet dramatically changed the staining pattern of IgG and ZO-1. In testes from control rabbits, ZO-1 was localized to the basal regions of seminiferous tubules, and IgG staining was excluded from the seminiferous tubules; both indicate that the BTB was functionally intact. In contrast, in testes from rabbits fed a cholesterol-enriched diet, more ZO-1 was present in the adluminal region of seminiferous tubules, and increasingly larger amounts IgG staining were present within the seminiferous tubule, indications that the integrity of BTB was disrupted.
Enhanced cholesterol uptake may affect endocytic trafficking of tight-junction proteins. Thus, we determined the extent to which the cholesterol-enriched diet affected tight-junction protein internalization. We demonstrated that cholesterol-enriched diet time-dependently altered the protein expression levels and distribution patterns of the tight-junction proteins ZO-1 and occludin. In control testes, these tight-junction proteins were observed only at the base of seminiferous tubules, and very little positive staining for endosomes was present. However, in rabbits fed the cholesterol-enriched diet, positive staining for tight-junction proteins became apparent in the adluminal side of seminiferous tubules, and the numbers and sizes of endosomes were markedly increased. Furthermore, in rabbits fed the cholesterol-enriched diet there was a temporally dependent increase in the codistribution of tight-junction proteins with endosomes. Consistent with our findings from immunostaining studies, we also demonstrated that cholesterol-enriched diet significantly increased protein levels of ZO-1 in endolysosome fractions. Thus, our findings suggest that elevated plasma cholesterol promotes endocytic accumulation of tight-junction proteins and thereby leads to a disrupted integrity of the BTB. Somewhat unexpectedly, we demonstrated that cholesterol-enriched diet also dramatically changed the distribution and increased the total protein levels of both ZO-1 and occludin. Such changes might be an adaptive change, because Sertoli cells increasingly synthesize tight-junction proteins and insert more tight-junction proteins in the adluminal side of seminiferous tubules when one attempts to stop leakage at the basal side of the seminiferous tubules. Such a notion is supported by our observation that in animals fed the cholesterol-enriched diet for 6 wk the amount of the lgG accumulated in testes reverted back to levels similar to those of animals fed the diet for 2 wk.
In summary, we demonstrated, in rabbits, that cholesterol-enriched diet promoted cholesterol accumulation within Sertoli cells, increased accumulation of tight-junction proteins in endocytic compartments, and disrupted the integrity BTB. Our findings suggest that elevated plasma levels of cholesterol disrupt the BTB integrity and contribute to male infertility by affecting endocytic trafficking of tight-junction proteins.
Therapeutic interventions to keep the BTB intact might help prevent and/or reverse some male infertility issues.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: D.H.M., L.H., and X.C. performed experiments; D.H.M., L.H., and X.C. analyzed data; D.H.M., O.G., J.D.G., and X.C. interpreted results of experiments; D.H.M. and X.C. prepared figures; D.H.M., O.G., L.H., J.D.G., and X.C. approved final version of manuscript; O.G., J.D.G., and X.C. conception and design of research; O.G., L.H., J.D.G., and X.C. edited and revised manuscript; X.C. drafted manuscript.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health Grants P30 GM-103329, R21 AG-043338, and R01 MH-100972 and by the Research Experiences for Medical Students program at UND.
REFERENCES
- 1.Bataineh HN, Nusier MK. Effect of cholesterol diet on reproductive function in male albino rats. Saudi Med J 26: 398–404, 2005. [PubMed] [Google Scholar]
- 2.Cheng CY, Mruk DD. The blood-testis barrier and its implications for male contraception. Pharmacol Rev 64: 16–64, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Comhaire FH, Mahmoud AM, Depuydt CE, Zalata AA, Christophe AB. Mechanisms and effects of male genital tract infection on sperm quality and fertilizing potential: the andrologist's viewpoint. Hum Reprod Update 5: 393–398, 1999. [DOI] [PubMed] [Google Scholar]
- 4.Dym M, Fawcett DW. The blood-testis barrier in the rat and the physiological compartmentation of the seminiferous epithelium. Biol Reprod 3: 308–326, 1970. [DOI] [PubMed] [Google Scholar]
- 5.Fijak M, Meinhardt A. The testis in immune privilege. Immunol Rev 213: 66–81, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Johnson MH. Changes in the blood-testis barrier of the guinea-pig in relation to histological damage following iso-immunization with testis. J Reprod Fertil 22: 119–127, 1970. [DOI] [PubMed] [Google Scholar]
- 7.Kolettis PN. Evaluation of the subfertile man. Am Fam Physician 67: 2165–2172, 2003. [PubMed] [Google Scholar]
- 8.Lewis-Jones DI, Richards RC, Lynch RV, Joughin EC. Immunocytochemical localisation of the antibody which breaches the blood-testis barrier in sympathetic orchiopathia. Br J Urol 59: 452–457, 1987. [DOI] [PubMed] [Google Scholar]
- 9.Maboundou JC, Fofana M, Fresnel J, Bocquet J, Le Goff D. Effect of lipoproteins on cholesterol synthesis in rat Sertoli cells. Biochem Cell Biol 73: 67–72, 1995. [DOI] [PubMed] [Google Scholar]
- 10.Maqdasy S, Baptissart M, Vega A, Baron S, Lobaccaro JM, Volle DH. Cholesterol and male fertility: what about orphans and adopted? Mol Cell Endocrinol 368: 30–46, 2013. [DOI] [PubMed] [Google Scholar]
- 11.Mascrez B, Ghyselinck NB, Watanabe M, Annicotte JS, Chambon P, Auwerx J, Mark M. Ligand-dependent contribution of RXRbeta to cholesterol homeostasis in Sertoli cells. EMBO Rep 5: 285–290, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morton D. The use of rabbits in male reproductive toxicology. Environ Health Perspect 77: 5–9, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakanishi Y, Shiratsuchi A. Phagocytic removal of apoptotic spermatogenic cells by Sertoli cells: mechanisms and consequences. Biol Pharm Bull 27: 13–16, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Padron RS, Mas J, Zamora R, Riverol F, Licea M, Mallea L, Rodriguez J. Lipids and testicular function. Int Urol Nephrol 21: 515–519, 1989. [DOI] [PubMed] [Google Scholar]
- 15.Papadopoulos V. Cholesterol homeostasis and infertility: the liver X receptor connection. Endocrinology 146: 2517–2518, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Poongothai J, Gopenath TS, Manonayaki S. Genetics of human male infertility. Singapore Med J 50: 336–347, 2009. [PubMed] [Google Scholar]
- 17.Ramirez-Torres MA, Carrera A, Zambrana M. [High incidence of hyperestrogenemia and dyslipidemia in a group of infertile men]. Ginecol Obstet Mex 68: 224–229, 2000. [PubMed] [Google Scholar]
- 18.Robertson KM, Schuster GU, Steffensen KR, Hovatta O, Meaney S, Hultenby K, Johansson LC, Svechnikov K, Soder O, Gustafsson JA. The liver X receptor-(beta) is essential for maintaining cholesterol homeostasis in the testis. Endocrinology 146: 2519–2530, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Russell L. Movement of spermatocytes from the basal to the adluminal compartment of the rat testis. Am J Anat 148: 313–328, 1977. [DOI] [PubMed] [Google Scholar]
- 20.Saez Lancellotti TE, Boarelli PV, Monclus MA, Cabrillana ME, Clementi MA, Espinola LS, Cid Barria JL, Vincenti AE, Santi AG, Fornes MW. Hypercholesterolemia impaired sperm functionality in rabbits. PLoS One 5: e13457, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Selva DM, Hirsch-Reinshagen V, Burgess B, Zhou S, Chan J, McIsaac S, Hayden MR, Hammond GL, Vogl AW, Wellington CL. The ATP-binding cassette transporter 1 mediates lipid efflux from Sertoli cells and influences male fertility. J Lipid Res 45: 1040–1050, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Shalaby MA, el-Zorba HY, Kamel GM. Effect of alpha-tocopherol and simvastatin on male fertility in hypercholesterolemic rats. Pharmacol Res 50: 137–142, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Slavis SA, Scholz JN, Hewitt CW, Black KS, Campbell RS, Patel M, Zimmerman J, Peake ML, Martin DC. The effects of testicular trauma on fertility in the Lewis rat and comparisons to isoimmunized recipients of syngeneic sperm. J Urol 143: 638–641, 1990. [DOI] [PubMed] [Google Scholar]
- 24.Vaid KS, Guttman JA, Babyak N, Deng W, McNiven MA, Mochizuki N, Finlay BB, Vogl AW. The role of dynamin 3 in the testis. J Cell Physiol 210: 644–654, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Vogl AW, Du M, Wang XY, Young JS. Novel clathrin/actin-based endocytic machinery associated with junction turnover in the seminiferous epithelium. Semin Cell Dev Biol 30C: 55–64, 2014. [DOI] [PubMed] [Google Scholar]
- 26.Wiebe JP, Tilbe KS. De novo synthesis of steroids (from acetate) by isolated rat Sertoli cells. Biochem Biophys Res Commun 89: 1107–1113, 1979. [DOI] [PubMed] [Google Scholar]
- 27.Wong CH, Cheng CY. The blood-testis barrier: its biology, regulation, and physiological role in spermatogenesis. Curr Top Dev Biol 71: 263–296, 2005. [DOI] [PubMed] [Google Scholar]
- 28.Wong EW, Mruk DD, Cheng CY. Biology and regulation of ectoplasmic specialization, an atypical adherens junction type, in the testis. Biochim Biophys Acta 1778: 692–708, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wong EW, Mruk DD, Lee WM, Cheng CY. Par3/Par6 polarity complex coordinates apical ectoplasmic specialization and blood-testis barrier restructuring during spermatogenesis. Proc Natl Acad Sci USA 105: 9657–9662, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xia W, Wong EW, Mruk DD, Cheng CY. TGF-beta3 and TNFalpha perturb blood-testis barrier (BTB) dynamics by accelerating the clathrin-mediated endocytosis of integral membrane proteins: a new concept of BTB regulation during spermatogenesis. Dev Biol 327: 48–61, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamamoto Y, Shimamoto K, Sofikitis N, Miyagawa I. Effects of hypercholesterolaemia on Leydig and Sertoli cell secretory function and the overall sperm fertilizing capacity in the rabbit. Hum Reprod 14: 1516–1521, 1999. [DOI] [PubMed] [Google Scholar]
- 32.Yan HH, Mruk DD, Lee WM, Cheng CY. Blood-testis barrier dynamics are regulated by testosterone and cytokines via their differential effects on the kinetics of protein endocytosis and recycling in Sertoli cells. FASEB J 22: 1945–1959, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]