Abstract
Whenever the visual stream is abruptly disturbed by eye movements, blinks, masks, or flashes of light, the visual system needs to retrieve the new locations of current targets and to reconstruct the timing of events to straddle the interruption. This process may introduce position and timing errors. We here report that very similar errors are seen in human subjects across three different paradigms when disturbances are caused by either eye movements, as is well known, or, as we now show, masking. We suggest that the characteristic effects of eye movements on position and time, spatial and temporal compression and saccadic suppression of displacement, are consequences of the interruption and the subsequent reconnection and are seen also when visual input is masked without any eye movements. Our data show that compression and suppression effects are not solely a product of ocular motor activity but instead can be properties of a correspondence process that links the targets of interest across interruptions in visual input, no matter what their source.
Keywords: compression, saccades, visual space
some of the strongest visual illusions occur at the time of saccadic eye movements (Melcher and Colby 2008). Objects presented briefly around saccade onset appear compressed in space and time (Ross et al. 1997; Lappe et al. 2000; Morrone et al. 2005; Hamker et al. 2008). Additionally, observers often fail to notice a displacement of the saccade target when it takes place during the execution of the saccade (Bridgeman et al. 1975; Deubel et al. 1996). It has been suggested that these effects reflect a dedicated mechanism that compensates for the retinal displacement of a target caused by the saccade (Melcher and Colby 2008).
We will suggest an alternative explanation for these effects, but first we outline the original findings. Several studies have examined the perceived locations of probes presented just before or after a saccade. When there are visible references in the display (Ross et al 1997; Lappe et al. 2000), the probes all appear shifted toward the saccade target, perisaccadic compression. In contrast, only shifts in the direction of the saccade are seen when tested in darkness (Honda 1989; Georg et al. 2008). Compression has also been observed orthogonal to the saccade path (Kaiser and Lappe 2004), suggesting probes from all directions will be dragged to the saccade target position. A motor origin of visual compression is suggested by the finding that compression magnitude correlates with saccade peak velocity (Ostendorf et al. 2007) and by the result that in antisaccades compression is centered on the saccade landing but not on the visual target position (Awater and Lappe 2004).
In addition to the mislocalization of probes at the time of a saccade, other studies have reported a surprising inability of observers to detect if the target itself has been displaced when the saccade lands, saccadic suppression of displacement (e.g., Bridgeman et al. 1975). However, the displacement again becomes visible if the shifted saccade target is not presented until 250 ms after the saccade (Deubel et al. 1996). This result suggested that the saccade target is used as the reference in transsaccadic perception of visual space. Niemeier et al. (2003, 2007) have suggested that saccadic suppression of displacement and the related spatial compression are explained by noise in the oculomotor system.
Finally, changes in the perceived duration of intervals and in the temporal order of successive events have also been reported at the time of a saccade (Morrone et al. 2005). In particular, short intervals of time between two successive visual stimuli occurring around the time of a saccade were underestimated. This temporal compression effect was specific to the visual modality, and the authors proposed a timing mechanism for visual stimuli that is modulated by saccade-related neural circuitry.
However, here we show that these three effects, spatial and temporal compression and displacement suppression, can be induced during ocular fixation when a visual mask disrupts retinal input and we suggest that it is the disruption, by a mask or by the masking caused by a saccade, and not the oculomotor system that causes all three. We previously observed that spatial compression can be created using a mask (Zimmermann et al. 2013a). We now extend this previous finding of spatial compression by also showing mask-induced temporal compression and suppression of displacement. In particular, we find that the magnitude of spatial and temporal compression and of suppression effects is comparable in fixation and saccade conditions suggesting that the effects arise from the operation of a more general mechanism that is not restricted to the oculomotor system. Although the findings we present are only correlational, they suggest a reconsideration of purely motor explanations. Surely, maintaining the identity of objects across visual discontinuities is a constant challenge for the visual system (Ullman 1979), one that arises whenever the eyes move or blink or whenever a distracting flash of light or a mask interrupts the visual input. We suggest that this correspondence mechanism, which links targets across disruptions, underlies these compression and suppression effects. Indeed, we show that the amount of spatial and temporal compression is greater when target features (orientation) match across an intervening saccade or mask.
Other authors have suggested a nonsaccade origin to spatial and temporal compression. The mislocalization observed during saccades can also be seen during fixation when retinal motion is replaced by visual background motion (Ostendorf et al. 2006; MacKay 1970; O'Regan 1984). Compression of interval duration can also occur during fixation when a luminance flicker is presented (Terao et al. 2008). We extend these previous results by proposing a common source for these compression effects (and the displacement suppression) based on the disruption caused by a mask or by a saccade and the correspondence process that links targets across the disruption.
The involvement of a correspondence process has been previously suggested by Cicchini et al. (2013). They proposed that perisaccadic compression reflects an attempt to connect presaccadic and postsaccadic visual features as two samples of the same object at a single location, bringing the locations into alignment to support visual stability across eye movements. We now show that this account extends beyond the saccade context they considered and show that it is part of a mechanism that bridges visual gaps in general.
METHODS
Spatial Compression
Participants.
Human subjects (5 participants, 3 females and 2 males, including 1 author, mean age: 31 yr) with normal or corrected-to-normal vision participated in the spatial compression experiment with informed consent. The experiments were carried out following the principles laid down in the Declaration of Helsinki. The study was approved by the ethics committee of the German Society of Psychology (DGPs).
Apparatus.
In the spatial compression experiment, subjects were seated 70 cm from a Samsung Sync-Master 2233 video monitor (Seoul, South Korea) with head stabilized by a chin- and headrest. The visible screen diagonal was 20 in., resulting in a visual field of 31.8 × 23.9°. Stimuli were presented on the monitor with a vertical frequency of 60 Hz at a resolution of 800 × 600 pixels. The stimuli were presented on a homogeneous, gray background (41.8 cd/m2). Eye movements were monitored by the EyeLink 2000 system (SR Research), which samples gaze positions with 2,000 Hz. Viewing was binocular, but only the dominant eye was recorded. The system detected start and end of a saccade when eye velocity exceeded or fell below 22°/s2 and acceleration was above or below 4,000°/s2.
Procedure.
FIXATION TRIALS.
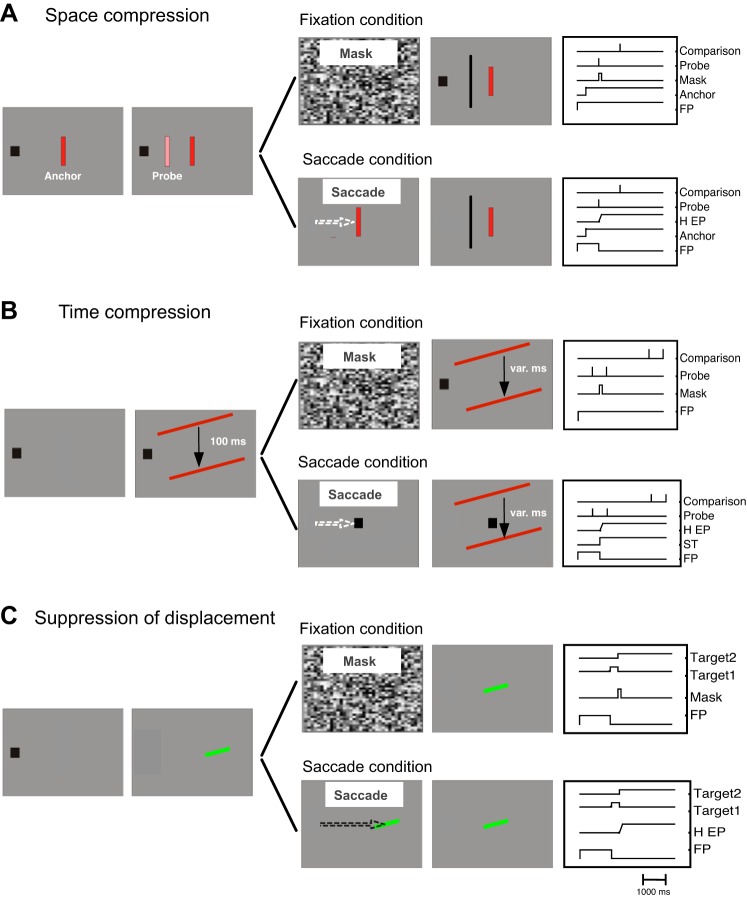
A trial started with the presentation of a fixation point 12° to the left of the screen center on the horizontal meridian of the monitor (see Fig. 1). Subjects were required to keep their gaze on the fixation point throughout the trial. After 1,000 ms plus a random delay between 0 and 500 ms, an anchor stimulus (red rectangle, size: 0.78 × 3.12°, luminance: 17.5 cd/m2) was presented at screen center on the vertical midline. The anchor stimulus remained visible for the rest of the trial. After 120 ms from the anchor's onset, a whole-field random texture mask was shown for 50 ms. At various times (−100, −50, 0, 50, and 100 ms relative to the mask onset), a probe stimulus was flashed for 16.6 ms. The probe stimulus had similar visual features (luminance: 57 cd/m2) as the anchor stimulus and was always presented on the horizontal meridian of the screen where its position varied across trials (−4, −2, 4, 6, and 8° relative to screen center). Subjects were instructed to judge the probe position with respect to a comparison stimulus that appeared 500 ms later, reporting whether the probe stimulus appeared to the left or to the right of the comparison. This procedure was tested in the “Same” condition with identical orientation of the anchor and the probe stimulus (see Fig. 1A). In the “Different” condition, the probe and anchor were presented with opposite orientation (−45, 45°). In the different condition, the comparison bar was shown with the same orientation as the probe stimulus. An adaptive staircase procedure (Best-Pest, 40 trials per threshold) was used to estimate the perceived position of the probe stimulus. Thresholds were measured in the Same and the Different conditions five times for each probe position (2 × 5 × 5 thresholds). Only trials in which gaze remained within a window of 2.5° around the fixation point were taken for analysis (∼97%).
Fig. 1.
A: experimental setup for the space compression experiment. B: experimental setup for the time compression experiment. C: experimental setup for the suppression of displacement experiment. FP, fixation point; H EP, horizontal eye position; ST, saccade target.
SACCADE TRIALS.
Saccade trials started with a fixation period. After 1,000 ms plus a random delay between 0 and 500 ms, an anchor stimulus was shown that had the same features as in fixation trials. Subjects had to perform a saccade to the center of the anchor as soon as it appeared. At various times around saccade onset a probe stimulus was presented for 16.6 ms. The probe was presented in the same spatial position as in fixation trials. After the execution of the saccade, subjects had to report the perceived probe position with a mouse cursor that appeared 500 ms after probe onset. Subjects were instructed to click in the bottom right corner of the screen in case they had not seen the probe. Saccade trials were tested in Same (identical orientation of anchor and probe, 4,464 trials in total) and Different (opposite orientation of anchor and probe, 3,038 trials in total) conditions.
Temporal Compression
Participants.
Five different subjects (3 females and 2 males, including 1 author, mean age: 32 yr) participated in the fixation trials and seven subjects (5 females and 2 males, including 1 author, mean age: 30 yr) in the saccade trials. All subjects had normal or corrected-to-normal vision and participated with informed consent. The experiments were carried out following the principles laid down in the Declaration of Helsinki.
Apparatus.
The same stimulation equipment as in the spatial compression experiment was used. Eye movements of the dominant eye were monitored by the IVIEW X Hi-Speed system, which samples gaze positions at 1,250 Hz. The system detected start and end of a saccade when eye velocity exceeded or fell below 40°/s.
Procedure.
FIXATION TRIALS.
A trial started with the presentation of a fixation point 12° to the left of the screen center on the horizontal meridian (see Fig. 1B). Subjects were required to maintain their gaze at the fixation point throughout the trial. After 1,000 ms plus a random delay between 0 and 500 ms, a bar (red rectangle, size: 31.8 × 0.8°, luminance: 32.5 cd/m2) was presented horizontally centered on the screen and 10° above the screen center for 16.6 ms. The bar was followed by a blank interval with a constant duration of 100 ms in all trials. At the end of the interval, a second bar (same features as first bar) was shown for 16.6 ms also horizontally centered but 10° below screen center. A whole-field random texture was presented for 50 ms at various times around that interval (−200, −150, −50, 50, and 100 ms relative the temporal center of the interval). After the second bar, a blank field was shown. After 2,000 ms, another pair of bars was presented (same features and durations as the 1st pair of bars). The interval between the two bars in the second pair varied from trial to trial (between 16.6 and 215.8 ms in 7 steps, constant stimuli). Subjects had to indicate by pressing the left or right arrow key whether the first or second interval appeared shorter. The bars were presented either with the Same or Different orientations. In the same conditions, all bars were presented either with 15° or with −15° orientation. In the different conditions, the upper bar could be oriented 15° and the lower bar −15° or vice versa. Again, only trials in which gaze remained within the fixation window of 2.5° were analyzed.
SACCADE TRIALS.
The procedure in saccade trials was similar to the fixation experiment except that at various times around presentation of the first bar of the first interval the fixation point was displaced to the center of the screen. Subjects had to make a saccade to the new position of the fixation point. After the saccade, subjects had to keep fixation at the new position throughout the rest of the trial. At the end of the trial subjects reported which interval appeared shorter. The Same (2,294 trials in total) and Different conditions (2,481 trials in total) (orientation of the bars) of the fixation trials were also present in the saccade trials.
Suppression of Displacement
Participants.
Nine subjects (5 females, 4 males, including 1 author, mean age: 29.2 yr) with normal or corrected-to-normal vision participated in the suppression of displacement experiment with informed consent. Four of them (1 male) were also tested in the visibility control experiment. The experiments were carried out following the principles laid down in the Declaration of Helsinki.
Apparatus.
Subjects were seated 57 cm from a Compaq P1220 CRT monitor (Houston, TX) with head stabilized by a chin- and headrest. The visible screen diagonal was 22 in., resulting in a visual field of 40.2 × 30.5°. Stimuli were presented on the monitor with a vertical frequency of 120 Hz at a resolution of 1,024 × 768 pixels. The stimuli were presented on a homogeneous, gray background (14 cd/m2). The experiment was programmed in Matlab (The MathWorks, Natick, MA) using the Psychophysics and Eyelink Toolbox extensions (Brainard 1997; Cornelissen et al. 2002; Pelli 1997). Eye movements were recorded using an EyeLink1000 desk-mounted eye tracker (SR-Research, Mississauga, ON, Canada) at a sampling rate of 1,000 Hz.
Procedure.
FIXATION TRIALS.
A trial started with the presentation of a fixation point at the screen center (see Fig. 1C). Subjects were required to maintain their gaze on this position throughout the trial and to judge the displacement of a target bar. After 1,000 ms, the fixation point disappeared and the first target (i.e., before displacement) was shown (light green bar: 14 cd/m2, x = 0.30, y = 0.48, subtending 1.4 × 0.32°, tilted either left or right, 20° angular from horizontal), either left or right from fixation on the horizontal meridian. The first target remained on screen for 192 ms and was followed either by the mask (50-ms duration, whole-field pattern of squares of 0.5° side length, each assigned a random gray), or its presentation was prolonged by another 50 ms (control condition). A blank period of 200 ms without any stimulus on screen then followed on half the trials. Finally, the second (randomly leftward or rightward displaced) target bar was shown until participants gave a manual response (left arrow key for a perceived leftward displacement, right arrow key for a perceived rightward displacement: later on recoded into “forward” and “backward” responses, see main text). Horizontal eccentricity of the two target bars from fixation was randomly chosen on each trial between 6 and 10° (center between the two stimuli). The distance between the two bars (i.e., the size of the displacement) was determined relative to their overall eccentricity: 4, 8, 18, or 32%. Given stimuli centered around the minimum eccentricity of 6°, this results in absolute displacements of 0.24, 0.48, 0.96, and 1.92°, and given stimuli centered around the maximum eccentricity of 10°, displacements are 0.40, 0.80, 1.60, and 3.20°. The second target bar was either presented with the same orientation or the opposite orientation as the first bar (randomly interleaved in the same block of trials) but had otherwise identical visual features. For each data point in the resulting psychometric functions (cumulative Gaussian, maximum-likelihood fit), we collected 16 trials from each participant. Trials were excluded (and participants received feedback on screen) if a saccade or blink was detected around the time of presentation of the two bars. Trials were also excluded if participants accidentally pressed the wrong key (all errors in total: 7.9% of trials).
VISIBILITY CONTROL TRIALS.
Visibility control trials followed the same procedure as fixation trials except that no blank trials were run and participants were not asked to judge the displacement on a given trial but whether the two target bars had the same (right arrow key) or different orientation (left arrow key).
SACCADE TRIALS.
Saccade trials started with a fixation period. After 1,000 ms, the first target bar (same features as in fixation trials) was shown either left or right from fixation on the horizontal meridian. Subjects had to perform a saccade towards it as soon as it appeared. As soon as a saccade was detected online, either the second (displaced) bar was shown directly or after a blank interval of 200 ms (online saccade detection criterion: gaze more than 1.5° away horizontally from its initial position at bar onset; online detection latency on average 236 ms after bar onset, mean saccade latency calculated post hoc with a 30°/s velocity and 8,000°/s2 acceleration criterion: 224 ms). The same stimulus eccentricities and displacement sizes were used as in fixation trials. The second target bar remained on screen until participants gave their response. The first and second target bar had either the same or different orientation. This amounted to 384 saccade trials for each participant considered in analysis: 16 trials for each combination of condition (no change-no blank, change-no blank, and no change-blank) and the eight displacement levels. The no mask control trials from the fixation trials also served as control trials for the saccade condition. Trials were excluded (feedback on screen) if 1) participants' gaze was lost by the eye tracker (e.g., as the result of a blink) around the time of presentation of the target bars, 2) the saccade did not start within 1.5° from fixation, 3) saccades were anticipatory (latency <100 ms), 4) saccades were launched in the wrong direction, 5) saccades were not executed within 1,000 ms, or 6) participants pressed the wrong key. Fixation errors occurred in 3.7% of trials (all other errors together: <2%).
RESULTS
Spatial Compression
To test spatial compression without eye movements we asked subjects to hold their gaze steadily on a fixation point throughout the whole trial (see Fig. 1A). After a variable delay (1,000–1,500 ms), an anchor bar was shown in the periphery until the end of the trial. The anchor bar in the fixation conditions served as the equivalent of a saccade target in saccadic conditions. After a constant delay of 120 ms, a visual mask appeared. At various times around the onset of the mask, a probe bar was flashed. Subjects were instructed to localize the probe relative to the position of a comparison bar (left or right, 2-alternative forced choice) that was flashed on following 500 ms after the mask. We compared these settings to localizations in a saccade condition where the anchor bar served as the saccade target and the probe was flashed at various times relative to the saccade. Subjects again localized the probe, this time by indicating its location with a mouse cursor that was presented 500 ms after the saccade. In both fixation and saccade conditions, the anchor and probe bars had either the same or different orientations with equal frequency. Note that although the anchor and probe were very similar, there was minimal confusion between the two as the anchor was clearly defined as the stationary stimulus, whereas the to-be judged probe was flashed.
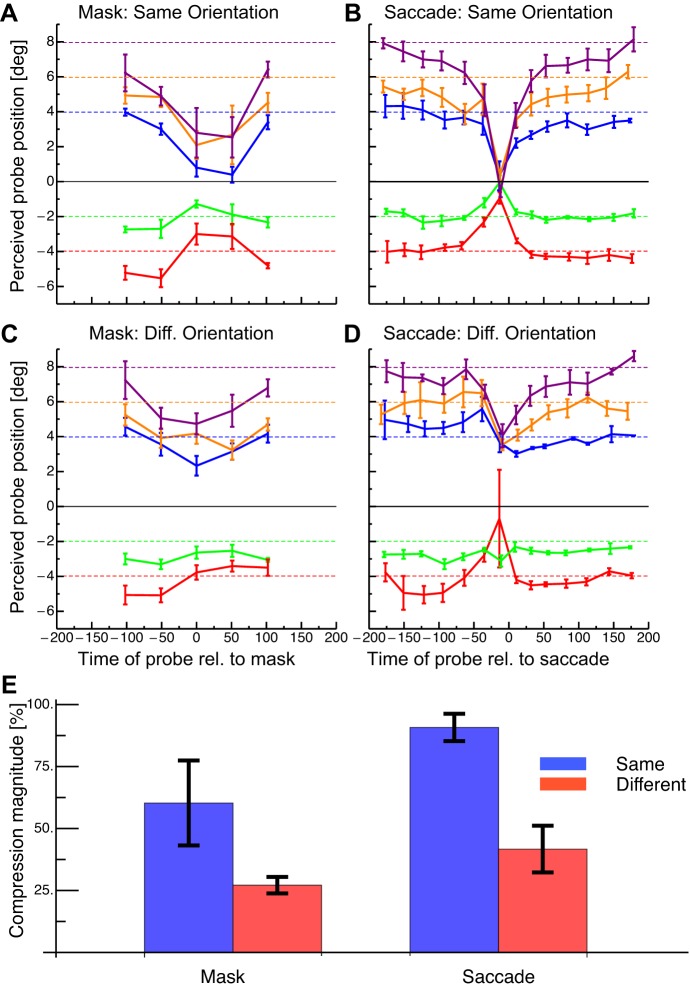
Figure 2A shows perceived probe position as a function of time to the onset of the mask in the fixation condition where anchor and probe had the same orientation. Data are averaged across subjects. Long before and after mask onset, localization was nearly veridical. At some positions, the reported probe location was biased towards the fovea. When probe stimuli were presented close in time to the mask onset, strong mislocalization was seen towards the anchor position (indicated by the black line at 0°). The observed pattern of mislocalization is very similar to that found in saccade trials (shown in Fig. 2B) where localization was again veridical long before and after saccade onset but was strongly mislocalized towards the anchor position when probe stimuli were presented close in time to saccade onset. The magnitude of mislocalization was reduced when the probe and anchor had different orientations in both the fixation (shown in Fig. 2C) and saccade conditions (shown in Fig. 2D) compared with the trials when probe and anchor had the same orientation (Fig. 2, A and B).
Fig. 2.
Average results of the space compression experiment. Negative time intervals on x-axis: probe before the mask/saccade and positive intervals on x-axis: probe after the mask. A: results in the fixation condition with same oriented probe and anchor stimuli. Dashed lines refer to physical probe positions. Error bars represent SE. B: results in the saccade condition with same oriented probe and anchor stimuli. Same conventions as in A. C: results in the fixation condition with different oriented probe and anchor stimuli. Same conventions as in A. D: results in the saccade condition with different oriented probe and anchor stimuli. Same conventions as in A. E: compression indexes from the fixation and saccade experiments with same and different oriented stimuli. Error bars represent SE.
We calculated a compression index by taking the difference between two values (for each of the 5 probe positions): the average position from the first bin long before mask or saccade onset minus the average position at the time of mask or saccade onset. This difference was divided by the physical distance between anchor and probe, and these indexes for the five probe positions were then averaged. In this average index, 100% means complete mislocalization of the probe towards the anchor and 0% means no mislocalization. This index was first calculated for each subject and before averaging across subjects. In the mask condition, there was larger mislocalization when the anchor and probe had the same orientation than when they had different orientations (shown in Fig. 2E). In the saccade condition, mislocalization was again larger for same orientations than for different orientations of the probe and anchor. A two-way ANOVA revealed that the mislocalization was larger in the same as opposed to the different orientation condition (df = 1, F = 15.25, P = 0.005). Mislocalization between the mask and saccade conditions did not differ significantly (df = 1, F = 5.01, P = 0.055). Critically, there was no significant interaction effect between the orientation and the mask/saccade condition (df = 1, F = 0.57, P = 0.471). Mislocalization was significantly >0 in all cases (t-tests: all P < 0.025). To check whether the difference in the response modality in the fixation and the saccade trials (localization relative to a bar vs. mouse pointing) had any influence, we ran fixation controls, in which three subjects reported the perceived bar position with a mouse pointer. All three subjects showed significantly stronger compression when the bars had the same rather than the different orientation (bootstrapped t-tests for the 3 subjects: P = 0.002, P = 0.021, P < 0.001).
Although there was no statistical difference between the compression indexes, the shape of the compression curves between the fixation and the saccade conditions differed: While the curves in the saccade condition had a sharp peak around saccade onset (Fig. 2, B and D), the curves in the fixation condition peaked for the whole duration of mask presentation (0–50 ms; Fig. 2, A and C). We had chosen a 50-ms interval for the presentation of our mask to mimic the approximate duration of the effects of the retinal displacement in the saccade conditions. Reduced sensitivity caused by a visual event, the saccade, can be categorized as masking (e.g., Castet et al. 2001). In this sense, the reduced sensitivity, “saccadic suppression,” during a specific time interval can also be achieved by masks without eye movements. However, other than that, we did not try to equate our mask to specifics of saccades (e.g., the velocity profile). Note further that for saccades, not only the retinal image displacement may disrupt the visual input, but it has also been suggested that an active mechanisms called saccade suppression, reduces visual sensitivity during saccades (Burr et al. 1999; Diamond et al. 2000; but see Castet et al. 2001). Saccade suppression is strongest at saccade onset (Diamond et al. 2000), which might explain the sharp peak at saccade onset in the saccade conditions.
Temporal Compression
In the temporal compression task, subjects maintained fixation while two bars were shown sequentially, one at the bottom and one at the top of the screen (see Fig. 1B). The interval between the onset of the two bars was always 100 ms. A visual mask was presented at various times relative to the temporal center of this interval. To determine the perceived temporal interval between the first two bars, a second pair of bars was shown 2,000 ms later at the same positions and with the same temporal order as the first pair. However, the interval between the onsets of the two bars of the second pair varied from trial to trial and subjects were instructed to report which interval appeared shorter.
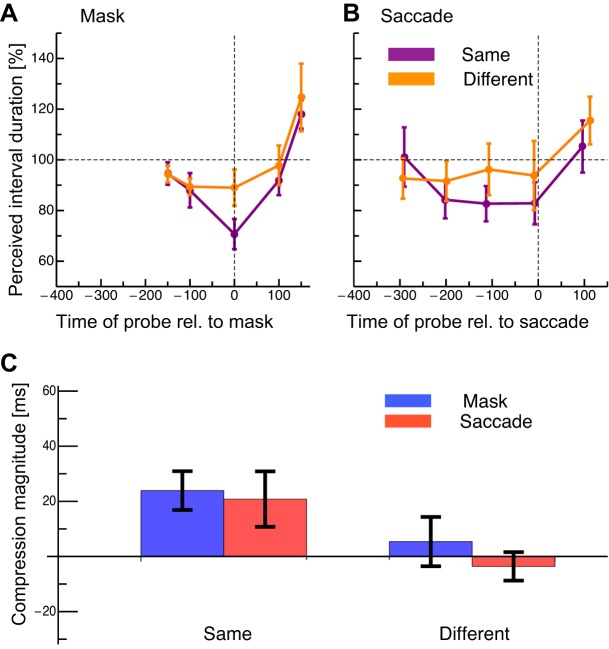
In the fixation condition when stimuli had the same orientation (see Fig. 3A, purple curve), the perceived duration was almost veridical when the interval center (i.e., the midpoint between the 1st and 2nd bar presentations) was −150 ms, i.e., before mask onset. However, when the center of the interval between the two bars appeared simultaneously with the mask, the apparent duration decreased. Long after the mask, the interval duration was overestimated. When the orientations of the two bar stimuli were different (see Fig. 3A, orange curve), the apparent duration of the interval was close to veridical when the interval was before or during the mask. As for the same-orientation stimuli, an overestimation was observed when the interval was presented long after the mask. In the saccade condition (see Fig. 3B), the misperception of the interval began earlier than in the fixation condition. When the stimuli had the same orientation (purple curve) and the interval was shown 300 ms before the saccade, the apparent duration was again veridical. When the interval came close to saccade onset, the perceived interval duration decreased. This underestimation of the interval remained relatively constant until the saccade onset fell in the center of the interval. When the interval was presented after the saccade, the perceived duration was again veridical. When the two bars had different orientations (orange curve), the apparent duration was very similar for saccades that occurred from −300 until 0 ms relative to the interval center. Only a slight underestimation of the interval was seen over this period. Saccades initiated after the interval produced an overestimation.
Fig. 3.
Average results of the temporal compression experiment. Values on x-axis indicate the time between the center of the probe interval and the onset of the mask or saccade (negative values, center of the probe interval preceded the mask/saccade; positive values, center of probe followed the mask/saccade). A: results in the fixation condition with same (shown in purple) and different (shown in orange) oriented stimuli. The dashed horizontal line shows the physical interval duration. Error bars represent SE. B: results in the saccade condition with same (shown in purple) and different (shown in orange) oriented stimuli. Same conventions as in A. C: compression indexes from the fixation (blue) and saccade (red) conditions for same and different oriented stimuli.
We calculated a temporal compression index by subtracting the apparent interval duration that occurred when the center of the interval was simultaneous with mask or saccade presentation from the apparent interval duration when the interval was long before the mask or saccade. We then divided this difference by 100 ms, the actual interval duration. This index was first calculated for each subject and then averaged across subjects. In this average index, 100% means complete compression of the interval, i.e., the two bars were perceived as appearing simultaneously, and 0% means veridical perception of the 100-ms interval. The compression index in the fixation condition where stimuli had the same orientation was comparable to that seen in previous studies with saccades (Morrone et al. 2005). Much less compression was found when stimuli had different orientations (see Fig. 3C). In the saccade condition, compression was seen when stimuli had the same orientation, again comparable to values seen in earlier studies, but compression was not significant when orientation differed. A two-way ANOVA revealed a significant main effect for the factor same-vs.-different orientation (df = 1, F = 9.24, P = 0.04) but not for the factor fixation-vs.-saccade (df = 1, F = 0.189, P = 0.67). No interaction effect was found (df = 1, F = 0.2, P = 0.67).
Suppression of Displacement
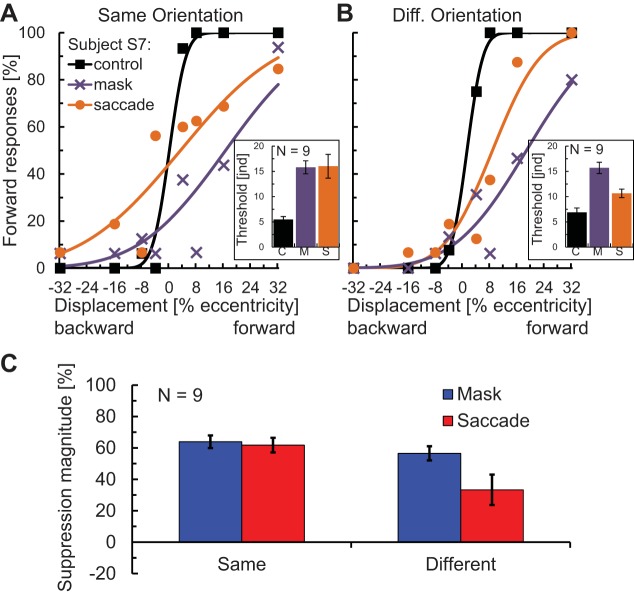
Because of the spatial compression effect of saccades (Ross et al. 1997; Lappe et al. 2000), we might expect, as suggested by Cicchini et al. (2013), that any displacement of the saccade target itself during the eye movement should be registered as smaller than it actually is. This would be a plausible explanation for the difficulty in detecting small target shifts during saccades, known as saccadic suppression of displacement (Bridgeman et al. 1975; Deubel et al. 1996). Since we observed spatial compression for a mask as well as for saccades both here and in a previous study (Zimmermann et al. 2013a), we next tested to what extent a mask would mimic the suppression of displacement effects seen with saccades (Zimmermann et al. 2013b) and whether this would depend on the correspondence between targets. We compared performance in a fixation condition without mask (control) against a fixation condition with a mask and a saccade condition (Fig. 1C). On each trial subjects reported whether the target moved away from initial fixation (forward) or back towards the initial fixation. Since Deubel et al. (1996) hadveshown that displacements are easier to detect if the target reappearance was delayed following the saccade, we tested both our mask and saccade conditions with and without a blank before the reappearance of the target. In addition, the target bar changed orientation during the displacement on half of the trials. Figure 4A shows the psychometric functions of a representative subject (S7), fit with cumulative Gaussian functions for the three conditions with same orientation. Figure 4B shows the fits for the same subject for the three conditions when the two bars (before and after displacement) had different orientations. Figure 4, insets, shows the mean Gaussian variance of the fits averaged across all nine participants. We took the Gaussian variance as an estimate of participants' discrimination threshold for forward vs. backward displacement (“just noticeable displacement”), where high variance indicates more suppression of displacement. In the same orientation conditions (Fig. 4A), thresholds in the saccade and mask condition were of comparable magnitude and both elevated compared with the control condition, indicating suppression of displacement. In the different orientation conditions (Fig. 4B), the threshold in the mask condition was also higher compared with control; however, the threshold in the saccade condition was only slightly elevated.
Fig. 4.
Results of the saccadic suppression of displacement experiment. Error bars in all graphs represent SE. A: psychometric functions of a representative subject and thresholds (variance of the underlying Gaussian; inset) averaged over the 9 participants for the fixation control, mask, and saccade conditions for same orientation/no blank trials. B: psychometric functions of the same subject and thresholds averaged over the 9 participants for different orientation/no blank trials. C: suppression indexes from the fixation and saccade experiments with same and different oriented stimuli. Same conventions as in A.
Similar to the two compression experiments, we also calculated a suppression of displacement index applying the following formula: suppression = (1 − control threshold/mask or saccade threshold) × 100%. In this index, 0% means no suppression of displacement (i.e., performance identical to control), and 100% means full suppression of displacement (i.e., no perceived displacement for any offset). The suppression indexes for the same vs. different orientation conditions are shown in Fig. 4C. A two-way ANOVA revealed a significant main effect for the factor same-vs.-different orientation (df = 1,8, F = 9.48, P = 0.015), a significant main effect for mask-vs.-saccade (df = 1,8, F = 7.28, P = 0.027), and a significant interaction between the two (df = 1,8, F = 7.72, P = 0.024). An orientation mismatch significantly reduced suppression in the saccade condition (df = 8, t = 2.90, P = 0.020) but not in the mask condition (df = 8, t = 1.61, P = 0.145). The suppression indexes were significantly greater than 0 in all cases (t-tests: all P < 0.009). The effect of the stimulus match has been seen in previous experiments on saccadic suppression of displacement. Specifically, suppression was reduced when the saccade target changed shape during the saccade (Demeyer et al. 2010; Tas et al. 2012).
A control experiment showed that the test bars remained visible even when masked. Participants were 98.0% accurate in reporting whether the second bar was the same or different orientation as the first when the mask was present and 97.0% correct when the mask was absent (df = 3, t = 0.98, ns), indicating that the first target was still visible when masked.
Furthermore, we checked whether our results show the well-known reduction of saccadic suppression of displacement when a blank is present (Deubel et al. 1996) and whether this reduction also happened with the mask instead of the saccade. A two-way ANOVA on these suppression indexes (data not shown) confirmed significantly more suppression on no blank compared with blank trials (no blank: 62.8% suppression, blank: −16.1% suppression, df = 1,8, F = 40.74, P < 0.001). There was no main effect of mask-vs.-saccade (df = 1,8, F = 1.84, P = 0.212) and no interaction (df = 1,8, F = 1.02, P = 0.343), indicating similar reductions in suppression of displacement with a blank for saccades and masks. We suggest that this blanking effect is a consequence of reduced compression when the postsaccadic probe is moved outside the temporal range of compression (for spatial compression and comparable conditions this range is ∼200 ms; Zimmermann et al. 2013a). This explanation for the blanking effect was proposed previously in the case of saccadic suppression of displacement by Cicchini et al. (2013), and here we show that it is a more general mechanism, visible as well when masking is the cause of the suppression of displacement.
DISCUSSION
All three effects, i.e., spatial and temporal compression as well as displacement suppression, have previously been proposed to be specific to the execution of a saccade. However, here we show that all three occurred at comparable strengths in the mask conditions without eye movements and in the saccade conditions. The results have several important implications: first, since the combination of a mask and an anchor stimulus can produce the effects, it seems plausible to suggest that the saccade also causes the spatial and temporal misperceptions due to its masking effect on the visual input stream. Second, the effects, rather than being specific to the oculomotor system, constitute a more general mechanism that modulates perception across discontinuities in the visual scene. Third, all effects, except fixational displacement suppression, showed a dependence on the correspondence between probe and anchor stimuli, thus suggesting that the compression and suppression effects are properties of an active matching mechanism that tries to establish object continuity across space and time.
In particular, we see these effects as properties of a process that matches corresponding objects across visual gaps in space and in time (Ullman 1979). If two objects are shown in rapid succession, this mechanism induces the phenomenon of apparent motion, interpreting the sequence as two samples of one moving object, even when their separation is too large to trigger responses in low-level motion detectors (Anstis 1980; Braddick 1980). We note that the correspondence match produces not only the impression of motion but may also contribute to the estimate of the distance traveled as the perceived length of an apparent motion trajectory is influenced by a number of factors even when the physical separation is fixed (Li et al. 2013). However, if the transient caused by the exchange of the two stimuli is degraded by a saccade or a visual mask, we suggest that this diminished or disorganized transient is interpreted as a diminished interstimulus distance, bringing the perceived location of the degraded stimulus closer to the position of the other. Interestingly, computational models like Kalman filters or Bayesian estimates (McNeilage et al. 2008) where the first stimulus affects the position estimate of the second stimulus also require correspondence between the two stimuli in order for the first to enter into the computation of the second. This is consistent with our finding of reduced compression and (saccadic) displacement suppression effects with different orientations of the two stimuli as in these cases the reduced correspondence would then reduce the contribution of the match to the position estimate. In the extreme, in the absence of any correspondence, we would expect no compression as the two stimuli would be taken as two separate items that could then be localized independently and veridically with no motion signal to contribute to the position estimates. We were unable to eliminate compression with the change of orientation we used but the apparent motion literature has shown that the correspondence match is quite tolerant to stimulus changes (Caelli et al. 1993; Cavanagh et al. 1989) so this is not unexpected. Nevertheless, in a competition motion paradigm, Green (1986) showed that orientation correspondence did support stronger apparent motion, consistent with our modulation of compression by orientation similarity.
We apply the correspondence match logic to time as well. Specifically, the match between the two bars in our temporal probe not only produces the impression of motion but may also contribute to the estimate of the time interval between the presentation of the two bars. Again, if the transient caused by the exchange of the two stimuli is degraded by a saccade or a visual mask, we suggest that this diminished or disorganized transient is interpreted as a diminished interstimulus duration, compressing the apparent time interval between them. Consistent with this interpretation we saw that time compression depended on object similarity. However, other stimulus-specific factors might also induce changes in the perception of interval duration: some authors suggest that durations are estimated based on a reference activity, like a neural clock, that is triggered by transient stimulus information (Buhusi and Meck 2005). Consequently, when transient information of one of the stimuli is degraded by a saccade or mask, the neural clock may weight its onset time by transient information of the second stimulus, thus reducing apparent interval duration. We saw an overestimation of the interval when it was presented slightly after saccade onset, which might be linked to perceptual chronostasis (Yarrow et al. 2001).
We had earlier suggested that the suppression of displacement effect might be a consequence of the compression due to the saccade (Cicchini et al 2013) or the mask, as the compression would reduce the effective displacement. According to this view, the motion transient of the target displacement is masked, because the offset of the first and the onset of the second stimulus fall in the perisaccadic masking period. Both stimuli are masked, reducing the motion transient between the two stimuli and reducing the estimate of their separation contributed by this motion signal. Compression is induced, where either the first target is attracted to the second or vice versa. We can therefore also see why the postsaccadic or postmask blank interval should lessen the suppression; it moves the temporal gap between first and second stimuli just outside the range where compression occurs (Cicchini et al 2013; Zimmermann et al 2013a). Again, this blank effect should hold for both saccades and masks as they have relatively similar time courses.
Other authors have already challenged the oculomotor origin of perisaccadic mislocalization and temporal compression. Two studies found that background motion in the absence of saccades shifted the perceived position of flashed stimuli (MacKay 1970; O'Regan 1984). Morrone et al. (1997) used a mirror, which rotated the visual scene at saccadic speed, thereby simulating the masking effects of natural saccades. Under this condition they only found a perceptual shift but no compression. However, Ostendorf et al. (2006) simulated saccades by shifting the screen image at saccadic speed and reported bidirectional compression shifts. Here we found that a simple mask is sufficient to induce the compression effects suggesting that compression occurs whenever stimulus transients are degraded, either by retinal or external motion or by a mask. Temporal compression in a fixation task has been reported earlier by Terao et al. (2008). They used dynamic luminance flicker in which the probe stimuli were embedded. The similarity between saccadic and mask-induced time compression again makes an oculomotor explanation unlikely.
An alternative to our generalized disruption proposal is that reflexive microsaccades produce the compression effects in the fixation paradigm since microsaccades have been shown recently to induce perisaccadic compression (Hafed 2013). However, this is unlikely for a number of reasons: first, compression during fixation is linked to the onset of the mask. If the mask had triggered a microsaccade, then compression would necessarily be delayed due to the latency of the microsaccade. Second, compression from microsaccades is small, a few minutes of arc, rather than the several degrees we report here. Finally, the compression would be focused on the “virtual” microsaccade target (Hafed 2013) quite close to fixation, not on the distant reference targets that act as attractors in our experiments.
Even if microsaccades could not explain our mask-induced compression effects, it might be that the appearance of the anchor triggers a saccade plan, which is then, due to the requirements of the task, not executed. This hidden saccade plan then might be responsible for the generation of the compression effects, as suggested by Atsma et al. (2014). If this were the case, however, we would not expect the presence or the timing of the mask to be of any great significance, whereas we find that it is (Fig. 2).
Finally, our mask-induced compression is focused on the attraction of the probe to the anchor stimulus. In contrast, saccade-induced compression is not always attracted toward the saccade target. In particular, Awater and Lappe (2004) showed that in an antisaccade paradigm, where a saccade has to be performed into the opposite direction of the saccade target, compression centers on the saccade landing position and not on the visual target position. This finding seems to contradict our hypothesis that compression is the result of an attempt to match the probe to an anchor stimulus. However, the antisaccade task can be solved either by inverting the motor vector in the opposite direction or by remapping the visual target signal to the new position. Evidence suggests that in the antisaccade task an inversion of the visual vector is involved (Sato and Schall 2003). In this view, the visual activity of the anchor stimulus would be remapped to the antiposition. The focus of compression then would be driven by the remapped anchor activity.
We have based our proposal of a common source for fixation and saccade effects on the similarity of results for the two conditions. We acknowledge that this similarity is not strong evidence in support of a common process. Indeed, the pattern of results for the two conditions does differ in time course (experiments 1 and 2) and sensitivity (experiment 3) so we cannot rule out the possibility that there is a contribution of the oculomotor system beyond the simple masking effect of the saccade. It may also be true that the differences in results between the saccade and mask conditions might be because the masking effects of saccades differs in many ways from the masking effect of our random noise fields. As mentioned before, the masking effects of saccades may stem from just the retinal image motion (Castet et al. 2001), but on top there might be an active mechanism reducing visual sensitivity, especially for transient signals, during saccades (Burr et al. 1999; Diamond et al. 2000). A third possibility is that perisaccadic compression consists of several components, one that is due to the masking effect of the saccade and one that is genuinely oculomotor in origin. Whatever the case, our results here show clearly that spatial compression is not necessarily saccade specific as it can be elicited by a mask with no eye movement. Also, the same point holds as well for temporal compression and displacement suppression.
Physiological findings of transsaccadic remapping processes (e.g., Duhamel et al. 1992) have motivated the interpretation of many of the compression and suppression of displacement effects, although more recent results (Zirnsak et al. 2014) make it difficult, for the moment, to propose any coherent link between the physiology and the behavior. Initially, perisaccadic spatial position shifts were interpreted as a signature of a transsaccadic remapping process (Ross et al. 2001). In particular, recordings in MT (Morris et al. 2012) show changes in responses of individual neurons that demonstrate perisaccadic mislocalization effects when these cell responses are used to decode stimulus position. In this view, the dynamic receptive field shifts, which occur transiently before saccade initiation (Duhamel et al. 1992), are the neurophysiological correlate of the perceptual shift of briefly presented perisaccadic visual probes (Hamker et al. 2011). However, the recent report (Zirnsak et al. 2014) has challenged both the original physiological claims of remapping (Duhamel et al 1992) and this interpretation of spatial compression. Zirnsak et al. (2014) found that frontal eye field (FEF) receptive fields, rather than shifting parallel to the saccade direction, converge towards the saccade target. This result suggests that receptive field shifts do not compensate for the upcoming saccade but instead cause a change in the target space during saccades. There is, as yet, no resolution of the conflict between these opposing physiological results concerning receptive field shifts at the time of the saccade. As for temporal compression, a physiologically based proposal has also been made based on recordings from the FEFs (Joiner et al. 2013). Probes were flashed in the center of each neuron's receptive field at different times just before a saccade. Although the different flashes covered time points stretching over a range of up to 80 ms, the responses to all the probes clustered very close together in time, typically over a range only one-third as large. This reduced time span of the responses relative to that of the probes was interpreted by the authors as a neural correlate of the saccade-induced temporal compression reported in behavioral experiments. Finally, in the case of saccadic suppression of displacement, studies again of FEF neurons have shown that the neural responses to a displacement occurring at the time of the saccade mimic the properties seen for human performance (Crapse and Sommer 2012). Neural modeling by Ziesche and Hamker (2014) has demonstrated how physiological responses in LIP can predict the saccadic suppression of displacement results.
Conclusion
We suggest that the masking (or equivalently, suppression) of transient onset, offset, and resulting apparent motion signals caused by the saccade is the source of space and time compression and also of saccade suppression of displacement. This masking, a reduction of visual sensitivity, may be a purely visual consequence of the saccade (e.g., caused by the retinal image motion; see Castet et al. 2001) or may also include contributions from active saccade-related perceptual suppression (Burr et al. 1999, Diamond et al. 2000). Whether or not the masking in the case of saccades is purely from visual factors is not critical for our argument that masking is a critical source of compression and displacement suppression effects. Our evidence for this claim comes from the very similar set of effects produced by a visual mask in the absence of saccade eye movements. Since these effects can be evoked by artificial masks displayed in the absence of saccades, they do not appear to be specifically linked to saccade programming. Researchers (Pola 2011; Burr and Morrone 2011; Richard et al. 2009) have suggested that interactions of spatial compression with the pre- and postsaccadic visual stimuli may well be in line with more complex transient receptive field adjustments to compensate for eye movements. Our findings of compression without saccades challenge accounts like these that restrict their explanations to sensorimotor processing.
GRANTS
This study was supported by a European Research Council Advanced Position (to P. Cavanagh) and the European Commission (F. Hamker, Spatial Cognition, FP7-FET Proactive, NeuroBio-Inspired Systems, No. 600785).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: E.Z., S.B., and P.C. conception and design of research; E.Z. and S.B. performed experiments; E.Z. and S.B. analyzed data; E.Z., S.B., and P.C. interpreted results of experiments; E.Z. and S.B. prepared figures; E.Z. and S.B. drafted manuscript; E.Z., S.B., G.R.F., and P.C. edited and revised manuscript; E.Z., S.B., G.R.F., and P.C. approved final version of manuscript.
REFERENCES
- Anstis SM. The perception of apparent motion. Philos Trans R Soc Lond B Biol Sci 290: 137–115, 1980. [DOI] [PubMed] [Google Scholar]
- Atsma J, Maij F, Corneil BD, Medendorp WP. No perisaccadic mislocalization with abruptly cancelled saccades. J Neurosci 34: 5497–5504, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awater H, Lappe M. Perception of visual space at the time of pro-and anti-saccades. J Neurophysiol 91: 2457–2464, 2004. [DOI] [PubMed] [Google Scholar]
- Braddick OJ. Low-level and high-level processes in apparent motion. Philos Trans R Soc Lond B Biol Sci 290: 137–115, 1980. [DOI] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spat Vis 10: 433–436, 1997. [PubMed] [Google Scholar]
- Bridgeman B, Hendry D, Stark L. Failure to detect displacement of the visual world during saccadic eye movements. Vision Res 15: 719–722, 1975. [DOI] [PubMed] [Google Scholar]
- Buhusi CV, Meck WH. What makes us tick? Functional and neural mechanisms of interval timing. Nat Rev Neurosci 6: 755–765, 2005. [DOI] [PubMed] [Google Scholar]
- Burr DC, Morgan MJ, Morrone MC. Saccadic suppression precedes visual motion analysis. Curr Biol 9: 1207–1209, 1999. [DOI] [PubMed] [Google Scholar]
- Burr DC, Morrone MC. Spatiotopic coding and remapping in humans. Philos Trans R Soc Lond B Biol Sci 366: 504–515, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caelli T, Manning M, Finlay D. A general correspondence approach to apparent motion. Perception 22: 185–192, 1993. [DOI] [PubMed] [Google Scholar]
- Castet E, Jeanjean S, Masson GS. “Saccadic suppression”: no need for an active extra-retinal mechanism. Trends Neurosci 24: 316–317, 2001. [DOI] [PubMed] [Google Scholar]
- Cavanagh P, Arquin M, von Grünau M. Interattribute apparent motion. Vision Res 29: 1197–1204, 1989. [DOI] [PubMed] [Google Scholar]
- Cicchini GM, Binda P, Burr DC, Morrone MC. Transient spatiotopic integration across saccadic eye movements mediates visual stability. J Neurophysiol 109: 1117–1125, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelissen FW, Peters EM, Palmer J. The Eyelink Toolbox: eye tracking with MATLAB and the Psychophysics Toolbox. Behav Res Methods Instrum Comput 34: 613–617, 2002. [DOI] [PubMed] [Google Scholar]
- Crapse TB, Sommer MA. Frontal eye field neurons assess visual stability across saccades. J Neurosci 32: 2835–2845, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demeyer M, Graef PD, Wagemans J, Verfaillie K. Object form discontinuity facilitates displacement discrimination across saccades. J Vis 10: 17, 2010. [DOI] [PubMed] [Google Scholar]
- Deubel H, Schneider WX, Bridgeman B. Postsaccadic target blanking prevents saccadic suppression of image displacement. Vision Res 6: 985–996, 1996. [DOI] [PubMed] [Google Scholar]
- Diamond MR, Ross J, Morrone MC. Extraretinal control of saccadic suppression. J Neurosci 20: 3449–3455, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duhamel JR, Colby CL, Goldberg ME. The updating of the representation of visual space in parietal cortex by intended eye movements. Science 255: 90–92, 1992. [DOI] [PubMed] [Google Scholar]
- Georg K, Hamker FH, Lappe M. Influence of adaptation state and stimulus luminance on peri-saccadic localization. J Vis 8: 15.1–11, 2008. [DOI] [PubMed] [Google Scholar]
- Green M. What determines correspondence strength in apparent motion? Vision Res 26: 599–607, 1986. [DOI] [PubMed] [Google Scholar]
- Hafed ZM. Alteration of visual perception prior to microsaccades. Neuron 77: 775–786, 2013. [DOI] [PubMed] [Google Scholar]
- Hamker FH, Zirnsak M, Calow D, Lappe M. The peri-saccadic perception of objects and space. PLoS Comput Biol 4: e31, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamker FH, Zirnsak M, Ziesche A, Lappe M. Computational models of spatial updating in peri-saccadic perception. Philos Trans R Soc Lond B Biol Sci 366: 554–571, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda H. Perceptual localization of visual stimuli flashed during saccades. Percept Psychophys 45: 162–174, 1989. [DOI] [PubMed] [Google Scholar]
- Joiner WM, Cavanaugh J, Wurtz RH. Compression and suppression of shifting receptive field activity in frontal eye field neurons. J Neurosci 33: 18259–18269, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser M, Lappe M. Perisaccadic mislocalization orthogonal to saccade direction. Neuron 41: 293–300, 2004. [DOI] [PubMed] [Google Scholar]
- Lappe M, Awater H, Krekelberg B. Postsaccadic visual references generate presaccadic compression of space. Nature 403: 892–895, 2000. [DOI] [PubMed] [Google Scholar]
- Li HH, Cavanagh P, Shim WM. Backward position shift in apparent motion. J Vis 14 pii: 16, 2013. [DOI] [PubMed] [Google Scholar]
- MacKay D. Mislocalization of test flashes during saccadic image displacements. Nature 227: 731–733, 1970. [DOI] [PubMed] [Google Scholar]
- McNeilage PR, Ganesan M, Angelaki DE. Computational approaches to spatial orientation: from transfer functions to dynamic bayesian inference. J Neurophysiol 100: 2981–2996, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melcher D, Colby CL. Trans-saccadic perception. Trends Cogn Sci 12: 466–473, 2008. [DOI] [PubMed] [Google Scholar]
- Morris AP, Kubischik M, Hoffmann KP, Krekelberg B, Bremmer F. Dynamics of eye-position signals in the dorsal visual system. Curr Biol 22: 173–179, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrone MC, Ross J, Burr DC. Apparent position of visual targets during real and simulated saccadic eye movements. J Neurosci 17: 7941–7953, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrone MC, Ross J, Burr D. Saccadic eye movements cause compression of time as well as space. Nat Neurosci 8: 950–954, 2005. [DOI] [PubMed] [Google Scholar]
- Niemeier M, Crawford JD, Tweed DB. Optimal transsaccadic integration explains distorted spatial perception. Nature 422: 76–80, 2003. [DOI] [PubMed] [Google Scholar]
- Niemeier M, Crawford JD, Tweed DB. Optimal inference explains dimension-specific contractions of spatial perception. Exp Brain Res 179: 313–323, 2007. [DOI] [PubMed] [Google Scholar]
- O'Regan JK. Retinal versus extraretinal influences in flash localization during saccadic eye movements in the presence of a visible background. Percept Psychophys 36: 1–14, 1984. [DOI] [PubMed] [Google Scholar]
- Ostendorf F, Fischer C, Gaymard B, Ploner CJ. Perisaccadic mislocalization without saccadic eye movements. Neuroscience 137: 737–745, 2006. [DOI] [PubMed] [Google Scholar]
- Ostendorf F, Fischer C, Finke C, Ploner CJ. Perisaccadic compression correlates with saccadic peak velocity: differential association of eye movement dynamics with perceptual mislocalization patterns. J Neurosci 27: 7559–7563, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelli DG. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spat Vis 10: 437–442, 1997. [PubMed] [Google Scholar]
- Pola J. An explanation of perisaccadic compression of visual space. Vision Res 51: 424–434, 2011. [DOI] [PubMed] [Google Scholar]
- Richard A, Churan L, Guitton DE, Pack CC. The geometry of perisaccadic visual perception. J Neurosci 29: 10160–10170, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross J, Morrone MC, Burr DC. Compression of visual space before saccades. Nature 386: 598–601, 1997. [DOI] [PubMed] [Google Scholar]
- Ross J, Morrone MC, Burr DC. Compression of visual space before saccades. Nature 10: 598–601, 1997. [DOI] [PubMed] [Google Scholar]
- Ross J, Morrone MC, Goldberg ME, Burr DC. Changes in visual perception at the time of saccades. Trends Neurosci 24: 113–121, 2001. [DOI] [PubMed] [Google Scholar]
- Sato TR, Schall JD. Effects of stimulus-response compatibility on neural selection in frontal eye field. Neuron 38: 637–648, 2003. [DOI] [PubMed] [Google Scholar]
- Tas AC, Moore CM, Hollingworth A. An object-mediated updating account of insensitivity to transsaccadic change. J Vis 12: 18, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terao M, Watanabe Yagi JA, Nishida S. Reduction of stimulus visibility compresses apparent time intervals. Nat Neurosci 11: 541–542, 2008. [DOI] [PubMed] [Google Scholar]
- Ullman S. The Interpretation of Visual Motion. Cambridge, MA: MIT Press, 1979. [Google Scholar]
- Yarrow K, Haggard P, Heal R, Brown P, Rothwell RC. Illusory perceptions of space and time preserve cross-saccadic perceptual continuity. Nature 414: 302–305, 2001. [DOI] [PubMed] [Google Scholar]
- Ziesche A, Hamker FH. Brain circuits underlying visual stability across eye movements-converging evidence for a neuro-computational model of area LIP. Front Comput Neurosci 8: 25, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann E, Fink G, Cavanagh P. Perifoveal spatial compression. J Vis 13: 21, 2013a. [DOI] [PubMed] [Google Scholar]
- Zimmermann E, Morrone MC, Burr DC. Spatial position information accumulates steadily over time. J Neurosci 33: 18396–18401, 2013a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zirnsak M, Steinmetz NA, Noudoost B, Xu KZ, Moore T. Visual space is compressed in prefrontal cortex before eye movements. Nature 507: 504–507, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]