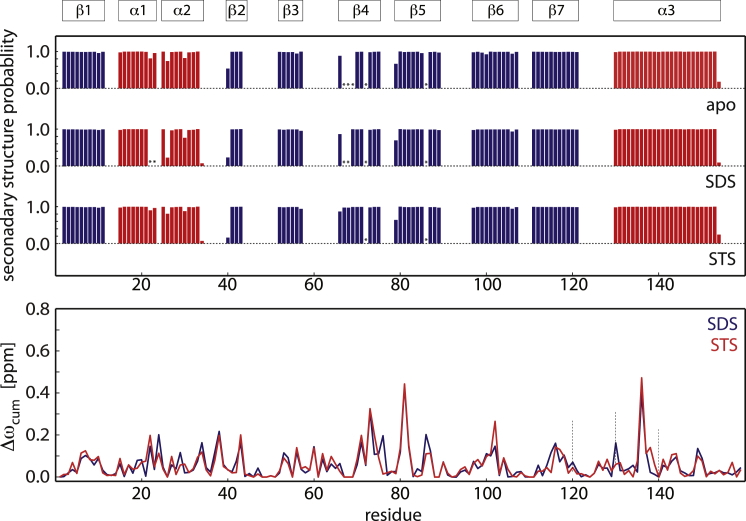
Figure 3.
NMR chemical shift data. Top: secondary structure of Bet v 1.0101 as defined by Gajhede et al. (14). Middle: TALOS+ secondary structure probabilities (blue, β-sheet; red, α-helix) of Bet v 1.0101 derived from 1HN, 15N, 13C’, 13Cα, and 13Cβ chemical shifts for the apo protein compared with complexes with SDS and STS. For residues that are marked by an asterisk, resonance assignments were not obtained. Bottom: cumulative chemical shift differences, Δωcum, between Bet v 1.0101 in the absence of ligand and Bet v 1.0101 in complex with SDS (blue) and STS (red), respectively, as a function of residue number. To see this figure in color, go online.