Abstract
Shiga toxin (Stx) is one of the most potent bacterial toxins known. Stx is found in Shigella dysenteriae 1 and in some serogroups of Escherichia coli (called Stx1 in E. coli). In addition to or instead of Stx1, some E. coli strains produce a second type of Stx, Stx2, that has the same mode of action as Stx/Stx1 but that is antigenically distinct. Because subtypes of each toxin have been identified, the prototype toxin for each group is now designated Stx1a or Stx2a. The Stxs consist of two major subunits, an A subunit that joins noncovalently to a pentamer of five identical B subunits. The A subunit of the toxin injures the eukaryotic ribosome, and halts protein synthesis in target cells. The function of the B pentamer is to bind to the cellular receptor, globotriaosylceramide, Gb3, found primarily on endothelial cells. The Stxs traffic in a retrograde manner within the cell, such that the A subunit of the toxin reaches the cytosol only after the toxin moves from the endosome to the Golgi and then to the endoplasmic reticulum. In humans infected with Stx-producing E. coli (STEC), the most serious manifestation of the disease, the hemolytic uremic syndrome or HUS, is more often associated with strains that produce Stx2a rather than Stx1a, and that relative toxicity is replicated in mice and baboons. Stx1a and Stx2a also exhibit differences in cytotoxicity to various cell types, bind dissimilarly to receptor analogs or mimics, induce differential chemokine responses, and have several distinctive structural characteristics.
Shiga toxin (Stx) is one of the most potent biological poisons known. Stx causes fluid accumulation in rabbit ileal loops, renal damage in mice, rabbits, greyhounds, and baboons, and is lethal to animals upon injection. However, humans encounter Stx as a consequence of infection with Shigella dysenteriae type 1 or certain serogroups of E. coli such as the O157:H7. There are two immunologically distinct groups of Stxs and this review will discuss toxin classification, structure and function, and the virulence associated with Stx-producing E. coli (STEC).
OVERVIEW
S. dysenteriae and the Stx were identified in the 19th century by Drs. Neisser and Shiga (1) and Conradi (2). Approximately 80 years later the same toxin (now called Stx1 to distinguish it from the toxin produced by S. dysenteriae) was found in a group of E. coli isolates. These bacteria caused bloody diarrhea and a serious sequelae, the hemolytic uremic syndrome (HUS), a condition characterized by thrombocytopenia, hemolytic anemia, and kidney failure (3, 4). Some E. coli strains were later shown to produce a highly related toxin, Stx2, that has the same mode of action as Stx/Stx1 but that is immunologically distinct. The Stxs (also known as Vero toxins, and previously as Shiga-like toxins) are a group of bacterial AB5 protein toxins of about 70 kDa that inhibit protein synthesis in sensitive eukaryotic cells. Protein synthesis is blocked by the Stxs through the removal of an adenine residue from the 28S rRNA of the 60S ribosome. This N-glycosidase activity of the toxin resides in the A subunit. The pentamer of identical B subunits mediates toxin binding to the cellular receptor globotriaosylceramide (Gb3). Additional commonalities between the Stx groups are that the subunit genes are encoded in an operon with the A subunit gene 5’ to that of the B subunit, that the stx operon is usually found within the sequence for an inducible, lysogenic, lambda-like bacteriophage, and that the toxins utilize a retrograde pathway to reach the cytoplasm. Differences between the two toxin groups include the fact that the genes for stx/stx1a are repressed by the Fur when high levels of iron are present (5–7), and that E. coli strains that encode stx2 are epidemiologically linked to more severe disease than those that carry stx1 (8, 9).
TYPING AND NOMENCLATURE
Although the prototype E. coli Stxs from each main group, Stx1 and Stx2 (now called Stx1a and Stx2a for distinction in the nomenclature from other toxin subtypes (10)), are the most common types found in association with disease from their respective groups, subtypes of each toxin exist, listed in Table 1. Toxin subtypes were originally only recognized when differences in biological activity and/or immunoreactivity could be demonstrated. However, as many new Stx-producing E. coli (STEC) were isolated and the toxin genes from those strains were sequenced, it became difficult to know if any differences found between the newly isolated gene and the prototype stx2a should result in the designation of another toxin subtype. Therefore, a phylogenetic analysis of stx sequences was undertaken and a PCR typing scheme developed that enables the assignment of a toxin to a particular subtype (10).
Table 1.
Prototype toxins and strains that produce those toxins
| Toxin type(s) | Prototype strain used for determination of stx subtypea |
Linked with serious human disease; difference(s) from prototype toxinb |
Reference(s) |
|---|---|---|---|
| Stx | 3818T | Yes | (133) |
| Stx1a | EDL933 (makes Stx1a and Stx2a) |
Yes | (113) |
| Stx1c | DG131/3 | No; immunologically distinct |
(134, 135) |
| Stx1d | MHI813 | No; immunologically distinct, less potent |
(136) |
| Stx2a | EDL933 (makes Stx1a and Stx2a) |
Yes | (113) |
| Stx2b (originally named VT-2d or Stx2d) |
EH250 | No; the B subunit gene was not detected by methods used to detect other sx2 B subunit genes |
(24) |
| Stx2c | 031 | Yes, less toxic to Vero cells and mice |
(137) |
| Stx2d (Stx2dact) |
C165-02 | Yes; more toxic after incubation with elastase, less toxic to Vero cells |
(138) |
| Stx2e | S1191 | No; binds to Gb4, associated with disease in pigs |
(139) |
| Stx2f | T4/97 | No; originally isolated in STEC from pigeons; immunologically distinct |
(140) |
| Stx2g | 7v | No; the stx2g gene is not amplified by primers specific for stx2a |
(141) |
More information about the prototypes strains may be found in reference (10).
Prototype toxin indicated in bold.
Stx/Stx1 subtypes
To date no variants of Stx as produced by Shigella have been described, but Stx is occasionally found in S. sonnei and type 4 S. dystenteriae (11, 12). Only two variants of Stx1a have been identified: Stx1c and Stx1d. Both Stx1c and Stx1d can be distinguished immunologically from Stx1 (13, 14). Stx1c and Stx1d are rarely found in human disease, and when associated with STEC isolated from patients, are linked with a mild disease course (15, 16).
Stx2a subtypes
The first Stx2a toxin variant identified as important for human disease, Stx2c, exhibits reduced cytotoxicity on Vero cells and reacts differently than Stx2a to some monoclonal antibodies (17). Another Stx2a variant, Stx2d (Stx2dactivatable), was identified because incubation with elastase from intestinal mucus increases the Vero cell cytotoxicity of the toxin (18, 19). The activatable Stx2d is associated with the most serious manifestation of STEC infection, the HUS (20). Both Stx2c and Stx2d show reduced cytotoxicity for Vero cells due to two amino acid (aa) differences in the B subunit but Stx2d is as toxic as Stx2a when injected into animals (21). Moreover, strains that produce Stx2d are highly virulent in a streptomycin-treated mouse model of infection (18, 22). In contrast, Stx2c is reported to show reduced toxicity compared to Stx2 when injected into mice (23). A different Stx2a subtype that was originally also named Stx2d, now known as Stx2b, is not activatable and is associated with mild disease (24, 25). Stx2e, Stx2f, and Stx2g are associated with animal STEC. Of the latter toxins, only Stx2e is associated with disease in the animal host; this toxin causes edema disease of swine, a rare, but serious neurological disorder that is frequently fatal (26).
SHIGA TOXIN STRUCTURE
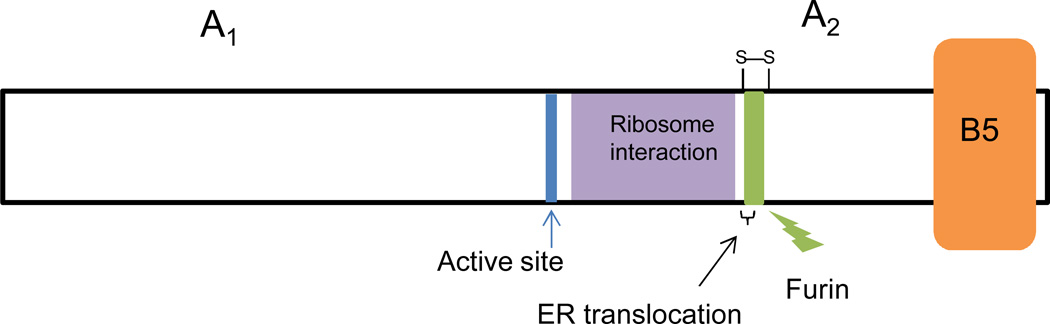
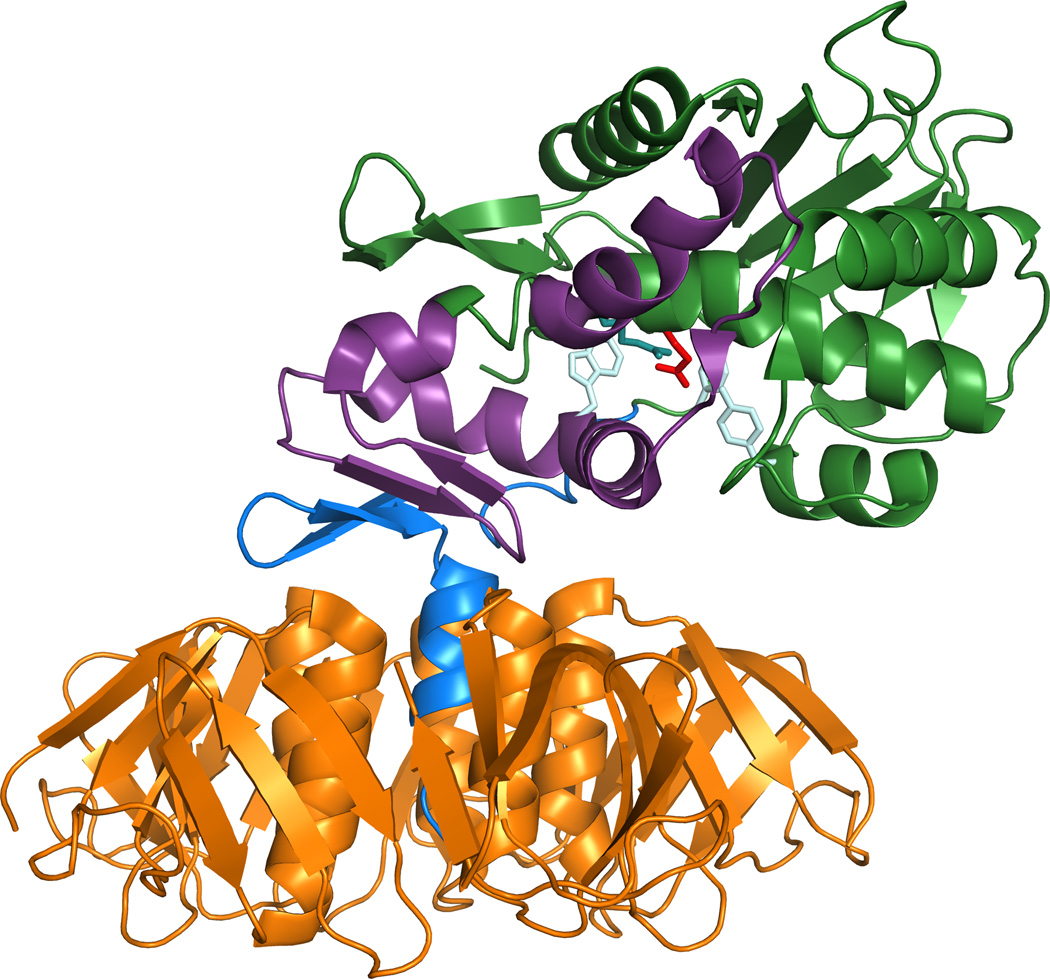
The mature A subunit of Stx/Stx1a consists of 293 aa while the Stx2a A chain is 4 aa longer at the C terminus. The active site aa of the toxin is the glutamic acid at position 167 (27). A trypsin sensitive region (aa 248–251) allows the A subunit to be cleaved asymmetrically into an A1 subunit and A2 peptide held together by a disulfide bridge, see illustration Fig. 1. The enzymatic activity of the toxin resides within A1 while the A2 peptide tethers A1 to the binding moiety, and further, threads through the B pentamer. For Stx2a the A2 peptide, in addition to maintaining holotoxin structure, appears to partially block the site three Gb3 binding site (discussed further below) (28), and for Stx2d, is crucial for the activation phenotype, as the final two aa of the A2 are cleaved when the toxin is treated with elastase (29). The pentamer of identical B subunits (each subunit = 69 aa for Stx/Stx1a, 71 aa for Stx2a) enables the toxin to find target cells that express surface Gb3. The crystal structures of the Stx B pentamer (30), Stx (31), Stx2a (28), Stx2a complexed with adenine (32), and the solution structures of the Stx1a B pentamer alone (33) or with the trisaccharide moiety from Gb3 (34) or a Gb3 analog (35) have been reported. A ribbon diagram of the crystal structure of Stx is shown in Fig. 2.
Figure 1. Cartoon representation of the Stx structure.
The active site glutamic acid is indicated as a vertical blue line, the ribosome interaction region is shown in purple, the protease (furin) sensitive site is depicted in green, and the B pentamer as an orange block. The disulfide bridge that connects the A1 subunit and the A2 peptide is shown above the protease sensitive site. A region important for translocation from the ER to the cytosol is indicated by a bracket. Not to scale.
Figure 2. Ribbon diagram of the Stx1 crystal structure.
The B pentamer is shown in orange and the A2 in blue. The majority of the A1 is depicted in green except for the region that interacts with the ribosome, which is shown in purple. The active residue 167 is red and other active-site side chains are pale blue. The A2 chain is medium blue and the B subunits are orange. The structure (1R4Q) was drawn with PyMOL Molecular Graphics System, Version 1.5.0 Schrödinger, LLC. Figure kindly provided by Dr. James Vergis.
Genetic analyses of the Stx A subunit
Besides the active site glutamic acid, colored red in Fig. 2., other A subunit aa residues that contribute to the full enzymatic function of Stx, colored pale blue in Fig. 2, include N75, Y77, Y114, R170, and W203 (W202 in Stx2) (27, 36–38). An analysis of truncated A1 fragments of Stx1a in yeast confirmed that residues within aa 1–239 are required for full enzymatic activity of the toxin, while the aa from 240–245 (and perhaps up to 251) are necessary for translocation of the A1 from the endoplasmic reticulum (ER) to the cytosol (39). The authors of the latter study hypothesized that it is the general structure of the region between aa residues 240 and 251that are recognized by anER mechanism that directs proteins from the ER into the cytosol. To define regions of Stx1a and the ribosome that interact, Gariepy’s group used yeast-two-hybrid and pull-down experiments and identified three ribosomal proteins that interact with the A1 subunit of the toxin and further showed that a conserved peptide from two of the ribosomal proteins inhibited Stx1a activity in vitro (40). They also found that the region from R170 to L233 (purple in Fig. 2) in Stx1a is important for ribosome interaction (41).
Stx AND CELL BINDING
As noted previously, the Stxs bind to the glycosphingolipid Gb3, a molecule comprised of a lipid or ceramide component and a trisaccharide of (α-gal(1→4)-β-gal(1→4)-β-glc) (42–44). Cell lines and mice deficient in Gb3 are insensitive to the Stxs (45–47). The normal cellular function of Gb3 is not known; however, individuals with excess Gb3, a condition called Fabry’s disease (48), exhibit kidney disease among other symptoms. When challenged with Stx2a, a mouse model of Fabry’s disease exhibits an elevated LD50 (49), perhaps due to mistargeting of the toxin to multiple sites of the body rather than the kidney, or to altered cellular trafficking due to changes in Gb3 subspecies populations. Stx1a and Stx2a interact with globotetraosylceramide (Gb4) in addition to Gb3, though weakly (50), while Stx2e binds Gb4 in preference to Gb3 (51, 52). An early report of a possible protein receptor for the Stxs (53) has not been substantiated in the literature.
Gb3 clusters within detergent insoluble portions of membranes called “lipid rafts” which also contain cholesterol and the cholera toxin receptor monosialotetrahexosylganglioside or GM1 (54, 55). The interaction of the Stx1a B subunit with HeLa cells causes a 2.5-fold increase in Gb3 present in the lipid raft domains (56), a result that suggests that cell binding by the B pentamer may promote stronger or additional toxin/cell interaction by recruiting more receptors to the rafts. Several reports indicate that the fatty acid chain length of the lipid component of Gb3 as well as the saturation state of the lipid also influence toxin binding (57, 58). The presence of cholesterol in the lipid rafts has been reported to increase toxin binding (50). Conversely, another group found no role for cholesterol in cell binding by the B subunit of Stx1a, but they did find that reductions in the cholesterol content of cells resulted in a decrease in uptake of the B pentamer (55). More recently, Lingwood’s group found that extraction of cholesterol from adult renal tissue sections enhanced Stx binding to glomeruli (59), a result that confirms the co-localization of Gb3 and cholesterol molecules. However, it may be difficult to dissect a possible role for cholesterol because Gb3 and cholesterol are both present in the lipid rafts, and alteration of either component may disrupt the integrity of the rafts. Taken together, these studies paint a picture of a complex interaction of the toxin with the host cell receptor, and suggest that the Stxs may interact differently with cells based on the nature of the receptor environment in the cell membrane.
The high affinity of the Stxs for Gb3 is likely due to the presence of at least two and up to three Gb3 binding sites per B monomer (two of the binding sites are present between adjacent monomers) as demonstrated by modelling studies and the crystal structure of the Stx B pentamer complexed with a Gb3 trisaccharide analog (35, 60). However, precise measurements of the binding affinity of the Stxs for Gb3 are difficult because Gb3 is not soluble. Therefore, soluble forms of the trisaccharide or trisaccharide analogs, or immobilized versions of the trisaccharide, Gb3, or Gb3 analogs are used to measure toxin/receptor interaction. When the interaction between the Stx1a B pentamer and the Gb3 trisaccharide was assessed in solution, binding site 2 (present within each monomer) was found to be the most highly occupied; site 1 was less engaged, and no interaction with site 3 was detected (34). Another study that also examined the interaction of the B pentamer with the Gb3 oligosaccharide component found that, at low concentrations of ligand, only site 2 was occupied though the authors of that study acknowledged that Stx binding is likely to be “polyvalent” at the cell surface (61). A mutational analysis of the three putative Gb3-binding sites in Stx1a confirmed the role of sites 1 and 2 for toxin/ receptor interaction (62). A mutation within site 3 (W34A) reduced binding of the holotoxin to Gb3, although the mutant toxin had the same overall affinity for Gb3 as did Stx1a. Another group found that Gb3-binding site 2 alone is not sufficient to confer high avidity binding of the toxin to Gb3, and that optimal binding to the receptor required all three sites (63). Although these latter studies on the number of functional Gb3 binding sites per toxin molecule were performed with Stx/Stx1a, the presence and primary importance of site 2 was confirmed for Stx2a (64). In addition, that site 1 contributes to the Vero cell toxicity phenotype of the Stx2a group is demonstrated by the fact that a single aa change in the Stx2d B subunit in the site 1 binding region renders that toxin as potent for Vero cells as Stx2a (21). Finally, a point in favor of the polyvalent nature of Stx binding is that Gb3 mimics with higher densities and clustering of the Gb3 trisaccharide (up to 18 copies) demonstrate higher affinities for both Stx1a and Stx2a (65, 66).
The question of how Stx enters the host from the site of pathogen colonization in the intestine remains, due to reports that colonic tissue lacks Gb3 (67). Macropinocytosis is a possible alternate mechanism for the uptake of Stxs into cells that do not express Gb3 (68). Polarized colonic T84 cells (which lack Gb3) nonetheless take up the Stx1a B pentamer in a way that is partially blocked by inhibitors of clathrin-dependent endocytic processes (68). However, such inhibition of the macropinocytic pathway does not reduce the amount of B subunit that is transcytosed through the monolayer, a finding that suggests that no matter which entry mechanism the Stx uses to enter these cells, the toxin can reach the basolateral side. That both Stx1a and Stx2a cross polarized T84 cells without disrupting the monolayer (46, 69, 70) or inhibiting protein synthesis has also been demonstrated (71). Therefore, it may be that some Gb3-negative intestinal cells allow systemic delivery of toxin in the absence of receptor. However, we and others have shown that incubation of intestinal cells with butyrate increases the sensitivity of intestinal cell lines to the Stxs (72, 73), and we further found that expression of Gb3 on intestinal cells is exquisitely sensitive to the removal of that gut metabolite (74). This latter finding suggests that previous measurements of Gb3 levels on intestinal tissues may be underestimates if butyrate was removed prior to Gb3 detection. In support of the possible presence of functional Gb3 receptors on intestinal tissue is the finding that incubation of pediatric intestinal explants with Stx2a showed cell extrusion from tissues taken from both ileum and colon (71). The damage observed in the latter study was specific as pre-incubation of the toxin with antiserum to Stx2a ameliorated the tissue injury. Furthermore, both Stx1a and Stx2a bind to Paneth cells when overlaid onto normal or inflamed pediatric duodenal tissues collected during endoscopy (75). We also found that that toxin overlaid into normal adult tissue binds colonic tissue (72). Finally, Malyukova et al. identified both Stx1a and Stx2a in intestinal tissues (epithelial cells and lamina propria) from O157-infected patients (68), although Gb3 could not be found in those intestinal epithelial cells (68). However, it may be that the Gb3 is masked in such tissues by cholesterol as described earlier, or that the expression of Gb3 was reduced by removal of butyrate when those samples were processed.
Stx/Gb3 interaction leads to uptake of the toxin/receptor complex through clathrin-dependent or –independent mechanisms. However, the clathrin-dependent process of entry appears to be the most common mode of uptake of the toxin/receptor complex (76). One perhaps surprising finding is that the A subunit of the Stx is involved in toxin uptake: more holotoxin is taken up via the endocytic pathway when the holotoxin binds to a cell as compared to B pentamer alone (77). The latter study illustrates the importance of confirming with the holotoxin findings based on the B pentamer alone. In clathrin-independent uptake, the Stx B subunit induces tubular-shaped invaginations within the HeLa cell plasma membrane, the function of which are not clear (78). Curiously, a mutation in Gb3 binding site 3 of the B subunit prevented formation of the invaginations (78), although the pentamer still bound to the cells.
RETROGRADE TRAFFICKING OF THE Stxs
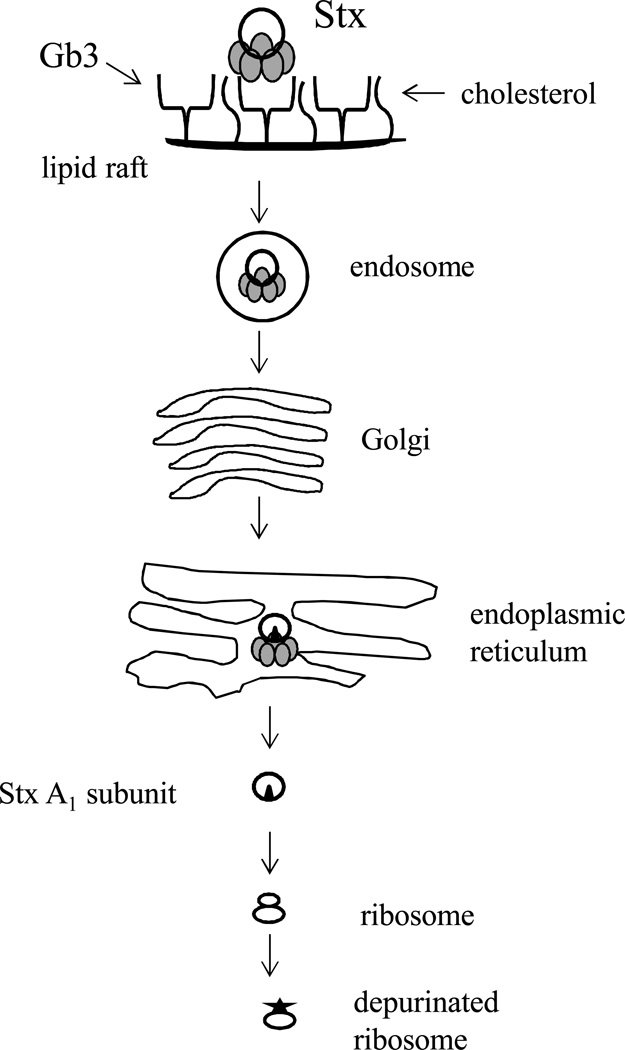
Stx was first shown to utilize a retrograde pathway to reach the cytosol more than 20 years ago in the human epidermal carcinoma cell line A431 which had been sensitized to the toxin by butyric acid treatment (79). An outline of the retrograde pathway used by the Stxs is shown in Fig. 3, and summarized as follows: after binding to Gb3, the toxin/receptor complex enters early endosomes, then traffics to the Golgi, and finally the ER (for a review see (80)). The protease sensitive site of the toxin A subunit is nicked either within the intestinal mucus (18) or within the Golgi (81); however, the nicked toxin retains the AB5 structure because of a disulfide bond between the A1 and A2 chains. The disulfide bond is reduced once the toxin gets into the ER, and only the toxin A1 subunit enters the cytosol. The Stx receptor, Gb3, is required for the toxin/receptor complex to move through the retrograde pathway, as toxin taken up by a nonreceptor-mediated mechanism or in cells depleted of glycosphingolipids do not reach the Golgi, though the complex does reach endosomes and possibly the ER (56, 70, 82). Many cellular proteins have now been identified that are involved in the Stx retrograde pathway (reviewed elsewhere (80, 83)). An unfolded toxin A1 chain exits the ER, apparently by subverting the ER-associated protein degradation (ERAD) pathway, to reach the target ribosomes (84, 85). The A1 then removes an adenine from the alpha-sarcin loop in the 28S ribosomal subunit. The injured ribosome no longer associates with elongation factor 1 (86, 87), and protein synthesis is halted. Although much of the work describing the retrograde pathway was done in epithelial cells, the primary target cell for the Stxs is the endothelial cell, discussed further below, and the Stx B pentamer has been shown to enter the retrograde pathway in mesangial and glomerular vascular endothelial cells as well (88).
Figure 3. An illustration of the retrograde pathway for Stxs.
The toxin binds to Gb3 within lipid rafts that contain cholesterol and that complex is internalized within an endosome. From the endosome the toxin traffics to the Golgi where it is nicked by furin if that nicking did not occur in the intestine. The nicked toxin moves to the ER where the disulfide bridge that keeps the A1 tethered to A2B5 is reduced. The A1 chain then enters the cytosol and removes an adenine residue from the 28S ribosome.
ACTIONS OF Stx IN TARGET CELLS
Numerous studies show that Stx inhibits protein synthesis in target cells and that active site mutants of Stx are no longer cytotoxic. Furthermore, a single molecule of Stx may be sufficient to kill a cell (85). Recently, however, the effects that the toxin has on cell signal transduction and immune modulation have begun to be explored (reviewed (89, 90)). Stx-mediated damage to the ribosome induces a response in cells called a “ribotoxic stress response” which is both pro-inflammatory and pro-apoptotic (see review (89)). The Stxs are also associated with an unfolded protein response that comes about through stress on the ER, and the result of which may also be apoptotic (reviewed (89, 91)). Evidence that Stx and STEC are associated with apoptosis came from rabbit studies in the mid-1980s (92, 93). Since that time, the molecular mechanisms of apoptosis due to the Stxs has been elucidated further (see review (91)).
Stx1a COMPARED TO Stx2a
Sx1a is about 10-fold more cytotoxic to Vero cells than Stx2a; however, the reverse is true in mice: Stx1a is 100–400-fold less lethal to mice than Stx2a, even though the toxins exhibit equivalent enzymatic activities/ng protein in vitro (94, 95). Epidemiological data from human disease indicate a stronger association with severe disease for STEC that produce Stx2a than Stx1a alone (96–98). So an important question within the STEC field is how to explain the paradox of the differential toxicity of the Stxs in vitro as compared to in vivo. We recently found that Stx2a is more toxic than Stx1a by the oral route in mice, data that supports the hypothesis that Stx2a is more potent from the gut and not just when injected intraperitoneally (99). One possible explanation for the differential toxicity of the toxins is that in contrast to epithelial cells such as Veros, the endothelial cells targeted in the HUS are more sensitive to Stx2a than Stx1a, and O’brig’s laboratory did find that renal microvascular endothelial cells obtained from human glomeruli are about 1000-fold more sensitive to Stx2a than Stx1a, although umbilical vein endothelial cells were equivalently sensitive (100). Since the Stxs are equally enzymatically active and they both bind Gb3, the question remains why different cell types exhibit differential sensitivity to the two Stx types. However, Gb3 does not exist in a homogeneous population within the cell, and it may be that the toxins do not bind equivalently to the Gb3 in target cells. An in vitro study with soluble forms of the trisaccharide moiety of Gb3 showed that Stx1a and Stx2a bind with similar affinity for that component of the receptor (64). Furthermore, Stx1a and Stx2a demonstrate similar affinities for renally-derived Gb3 as measured by enzyme-linked immunosorbent assay (ELISA), though by thin-layer chromatography Stx2a exhibits less binding than Stx1a (101). Examinations of toxin interaction with immobilized trisaccharides suggest that Stx1a has a higher affinity for the carbohydrate than does Stx2a (50, 102). Other experiments that examined the capacity of the toxins to bind Gb3 analogs showed that Stx1a and Stx2a do exhibit differential binding to those analogs in vitro (103), but whether similar analogs exist or act functionally as receptors in vivo has not been demonstrated.
Membrane cholesterol may also mask a portion of the potential Stx1a-binding Gb3 pool in Vero cell membranes (104) and adult renal glomeruli sections as mentioned previously (59), and incubation of Stx1a but not Stx2a with Gb3, cholesterol, and phosphatidylcholine partially neutralizes the cytotoxicity of Stx1a but not Stx2a (103). Stx2a, in contrast, is neutralized by human serum amyloid component P and lipopolysaccharide (LPS) from O107 or O117 E. coli (105, 106). The fact that the above compounds neutralize either Stx1a or Stx2a specifically, and presumably by preventing the toxins from binding to the cell, supports the hypothesis that the B pentamers of these toxins bind differently to cells. The Stx1a B pentamer is more stable than the comparable binding moiety of Stx2a (64) and that difference was shown to be at least partially due to a single aa residue within the B subunit (107). A study that examined the interaction of Stx1a and Stx2a with lipid rafts and subsequent intracellular trafficking in Vero cells showed that although both toxins associated with lipid rafts, Stx2a was also found in detergent-soluble (non-lipid raft) fractions of the membrane (108). In addition, some of the internalized Stx2a, but not Stx1a, co-localized with the cell surface marker transferrin, a finding that suggests that Stx2a is endocytosed from different areas of the membrane than Stx1a. However, both toxins localized to the Golgi after 1 h incubation with the Vero cells, although there was a suggestion that Stx2a exited from the Golgi at a slower rate than Stx1a.
At the level of the immune response to the toxins, Stx1a and Stx2a elicit differential chemokine responses from human umbilical vein endothelial cells (109) and a renal tubular epithelial cell line, HK-2 (110). The HK-2 cells are more sensitive to Stx1a than Stx2a and Stx2a-treatment induces expression of the macrophage chemoattractants macrophage inflammatory protein-1α (MIP-1α) and MIP-1β (110). However, in a baboon intoxication model, there was no change in MIP-1β in response to either Stx1a or Stx2a (111). Nonetheless, in that baboon model many characteristics of the HUS are observed, and Stx2a is lethal at lower doses than Stx1a, although the kidney injury takes longer to develop in the Stx2a-treated animals.
Taken together these studies on the differences between Stx1a and Stx2a indicate that the interaction between each toxin and the receptor is complex and influenced by several factors. Four distinctions between the Stx and Stx2a crystal structures that may contribute to the known biological differences exhibited by these toxins as discussed above are as follows: i) the Stx active site is partially blocked by the N-terminal region of the A2 peptide; ii) The Stx2a A2 C terminus has a more ordered structure than that of Stx; iii) Gb3-binding site 2 has a different conformation in each of the toxins; and, iv) Gb3-binding site 3 is partially blocked by the C-terminal two aa of the Stx2a A2 peptide (28).
Stx ASSOCIATION WITH DISEASE
HUS cases were reported in the literature as early as the 1950s, though the cause was unknown. An understanding of the origins of infection-associated HUS was also complicated by the fact that some cases of the HUS are of genetic origin (see review (112)). However, in 1983 bloody diarrhea and the HUS were linked to certain serogroups of E. coli and O’Brien et al. found that those strains produced a toxin related to the Stx of S. dysenteriae (3, 4, 113). The strong association between the HUS and Stx2a was underscored in the largest outbreak of the HUS in Germany in 2011 in which more than 800 cases of the disease were identified (114). The surprise from German outbreak was that although the implicated strain was an E. coli, unlike typical STEC, the epidemic isolate also encoded virulence factors associated with enteroaggregative E. coli (EAEC). EAEC had only rarely been associated with the HUS previously, and in each of those cases the implicated isolate was found to carry an Stx gene.
In animal models, purified Stx in the absence of detectable LPS is linked to kidney damage and death (99, 115). S. dysenteriae mutants that lack stx caused a milder dysentery in monkeys and produced only a trace of blood in stool compared to the wild-type isolate (116). Furthermore, Stx can be detected in the kidneys of some patients (117–119). Animal models further show that antibodies to the Stxs are protective, and humanized versions of those antibodies have completed Phase I trials (120, 121).
STEC Pathogenesis
STEC pathogenesis requires ingestion of the bacteria in contaminated food or water, though the organism may occasionally be passed person-to-person. To cause disease the organism colonizes the intestine, a process that likely includes attachment as well as proliferation. That STEC that carry the locus of enterocyte effacement (LEE) use the adhesin intimin (encoded by the eae gene) to adhere within the intestine has been shown in mouse and pig models (122, 123). In addition, STEC that are eae positive are more likely to cause disease than intimin-negative strains even in non-O157 strains (8, 124). We recently demonstrated in mice that a high fiber diet may influence the development of disease after O157 infection (74, 125). The influence of the higher fiber diet was two-fold and was associated with the production of butyrate within the intestine: mice on a high fiber diet were colonized to a greater degree than mice on a low fiber diet perhaps as a consequence of reduced overall levels of competitive native Escherichia species, and increased levels of Gb3 were observed in both the intestine and kidney.
After colonization with STEC, the innate proinflammatory response may initially be suppressed in those infected with strains that are LEE+ (see review (90)), a conclusion supported by the fact that STEC infection is generally not an inflammatory diarrhea. In the intestine the STEC elaborate Stx(s), and the patient may develop hemorrhagic colitis, the pathogenesis of which is not clearly understood. For systemic consequences, the Stx(s) enter the host from the intestine and reach target endothelium cells in the kidney and in some cases, the central nervous system. The Stx(s) damage cells directly and cause apoptosis as described above. The Stx(s) and likely LPS also elicit cytokines and other immune mediators within the kidney and from monocytes (for a review see (90)). Endothelial cells within the glomeruli become damaged and may die and detach from the membrane. Extrusion of the cell exposes collagen, normally not present within the blood vessel, and platelets become activated. The presence of activated platelets encourages fibrin deposition and leads to the development of a thrombus and promotes a coagulation cascade. Recent studies suggest that Stx-treatment of endothelial cells can induce the expression of factors that may stabilize clot formation even in the absence of cell death or extrusion (see reviews (126, 127)). Such damaged endothelial cells also become a site for the adhesion of leukocytes, the consequence of which is additional damage to the kidney (128). The localized prothrombotic environment in the glomerulus leads to the reduction of platelets in the serum (thrombocytopenia), hemolytic anemia, and renal damage, the triad of the HUS (for reviews see (126, 129, 130)). Altered complement levels have been found in patients with STEC HUS (131, 132), but whether those changes are part of the etiology of the disease or occur as a consequence is not clear (see (127) for review of possible role for complement in STEC HUS).
SUMMARY
The Stxs are potent poisons associated with bloody diarrhea and the potentially severe disease, the HUS. The Stx(s) act as ribotoxins that halt protein synthesis within the cell and induce apoptosis, but can also prompt altered gene/protein expression in epithelial cells, endothelial cells, monocytes, and mesangial cells. The consequence of exposure to E. coli that elaborate one or more Stx can range from asymptomatic infection to bloody diarrhea and, in some patients, the HUS. In the research arena, the Stxs have been exploited beautifully to interrogate mechanisms of retrograde transport, and to localize sites of Gb3 expression, and, more recently, the possible function of tubular invaginations in membranes.
ACKNOWLEDGMENTS
A special thank-you to Alison D. O’Brien for review of this chapter. This work was supported by R37 AI020148 and 2U54 AI057168.
References
- 1.Trofa AF, Ueno-Olsen H, Oiwa R, Yoshikawa M. Dr. Kiyoshi Shiga: discoverer of the dysentery bacillus. Clin Infect Dis. 1999;29:1303–1306. doi: 10.1086/313437. [DOI] [PubMed] [Google Scholar]
- 2.Conradi H. Uber Iosliche, durch asptische Autolyse erhaltene Giftstoffe vonRuhr- und Typhus-Bazillen. Dtsch. Med. Wochenschr. 1903;29:26–28. [Google Scholar]
- 3.Karmali MA, Steele BT, Petric M, Lim C. Sporadic cases of haemolytic-uraemic syndrome associated with faecal cytotoxin and cytotoxin-producing Escherichia coli in stools. Lancet. 1983;1:619–620. doi: 10.1016/s0140-6736(83)91795-6. [DOI] [PubMed] [Google Scholar]
- 4.O'Brien AO, Lively TA, Chen ME, Rothman SW, Formal SB. Escherichia coli O157:H7 strains associated with haemorrhagic colitis in the United States produce a Shigella dysenteriae 1 (SHIGA) like cytotoxin. Lancet. 1983;1:702. doi: 10.1016/s0140-6736(83)91987-6. [DOI] [PubMed] [Google Scholar]
- 5.Calderwood SB, Mekalanos JJ. Iron regulation of Shiga-like toxin expression in Escherichia coli is mediated by the fur locus. J Bacteriol. 1987;169:4759–4764. doi: 10.1128/jb.169.10.4759-4764.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dubos RJ, Geiger JW. Preparation and properties of Shiga toxin and toxoid. J Exp Med. 1946;84:143–156. [PubMed] [Google Scholar]
- 7.O'Brien AD, LaVeck GD, Thompson MR, Formal SB. Production of Shigella dysenteriae type 1-like cytotoxin by Escherichia coli . J Infect Dis. 1982;146:763–769. doi: 10.1093/infdis/146.6.763. [DOI] [PubMed] [Google Scholar]
- 8.Luna-Gierke RE, Griffin PM, Gould LH, Herman K, Bopp CA, Strockbine N, Mody RK. Outbreaks of non-O157 Shiga toxin-producing Escherichia coli infection: USA. Epidemiol Infect. 2014:1–11. doi: 10.1017/S0950268813003233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friedrich AW, Bielaszewska M, Zhang WL, Pulz M, Kuczius T, Ammon A, Karch H. Escherichia coli harboring Shiga toxin 2 gene variants: frequency and association with clinical symptoms. J Infect Dis. 2002;185:74–84. doi: 10.1086/338115. [DOI] [PubMed] [Google Scholar]
- 10.Scheutz F, Teel LD, Beutin L, Pierard D, Buvens G, Karch H, Mellmann A, Caprioli A, Tozzoli R, Morabito S, Strockbine NA, Melton-Celsa AR, Sanchez M, Persson S, O'Brien AD. Multicenter evaluation of a sequence-based protocol for subtyping Shiga toxins and standardizing Stx nomenclature. J Clin Microbiol. 2012;50:2951–2963. doi: 10.1128/JCM.00860-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta SK, Strockbine N, Omondi M, Hise K, Fair MA, Mintz E. Emergence of Shiga toxin 1 genes within Shigella dysenteriae type 4 isolates from travelers returning from the Island of Hispanola. Am J Trop Med Hyg. 2007;76:1163–1165. [PubMed] [Google Scholar]
- 12.Beutin L, Strauch E, Fischer I. Isolation of Shigella sonnei lysogenic for a bacteriophage encoding gene for production of Shiga toxin. Lancet. 1999;353:1498. doi: 10.1016/S0140-6736(99)00961-7. [DOI] [PubMed] [Google Scholar]
- 13.Zhang W, Bielaszewska M, Kuczius T, Karch H. Identification, characterization, and distribution of a Shiga toxin 1 gene variant (stx(1c)) in Escherichia coli strains isolated from humans. J Clin Microbiol. 2002;40:1441–1446. doi: 10.1128/JCM.40.4.1441-1446.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohmura-Hoshino M, Ho ST, Kurazono H, Igarashi K, Yamasaki S, Takeda Y. Genetic and immunological analysis of a novel variant of Shiga toxin 1 from bovine Escherichia coli strains and development of bead-ELISA to detect the variant toxin. Microbiol Immunol. 2003;47:717–725. doi: 10.1111/j.1348-0421.2003.tb03441.x. [DOI] [PubMed] [Google Scholar]
- 15.Friedrich AW, Borell J, Bielaszewska M, Fruth A, Tschape H, Karch H. Shiga toxin 1c-producing Escherichia coli strains: phenotypic and genetic characterization and association with human disease. J Clin Microbiol. 2003;41:2448–2453. doi: 10.1128/JCM.41.6.2448-2453.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar A, Taneja N, Kumar Y, Sharma M. Detection of Shiga toxin variants among Shiga toxin-forming Escherichia coli isolates from animal stool, meat and human stool samples in India. J Appl Microbiol. 2012;113:1208–1216. doi: 10.1111/j.1365-2672.2012.05415.x. [DOI] [PubMed] [Google Scholar]
- 17.Schmitt CK, McKee ML, O'Brien AD. Two copies of Shiga-like toxin II-related genes common in enterohemorrhagic Escherichia coli strains are responsible for the antigenic heterogeneity of the O157:H- strain E32511. Infect Immun. 1991;59:1065–1073. doi: 10.1128/iai.59.3.1065-1073.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Melton-Celsa AR, Darnell SC, O'Brien AD. Activation of Shiga-like toxins by mouse and human intestinal mucus correlates with virulence of enterohemorrhagic Escherichia coli O91:H21 isolates in orally infected, streptomycin-treated mice. Infect Immun. 1996;64:1569–1576. doi: 10.1128/iai.64.5.1569-1576.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kokai-Kun JF, Melton-Celsa AR, O'Brien AD. Elastase in intestinal mucus enhances the cytotoxicity of Shiga toxin type 2d. J Biol Chem. 2000;275:3713–3721. doi: 10.1074/jbc.275.5.3713. [DOI] [PubMed] [Google Scholar]
- 20.Bielaszewska M, Friedrich AW, Aldick T, Schurk-Bulgrin R, Karch H. Shiga toxin activatable by intestinal mucus in Escherichia coli isolated from humans: predictor for a severe clinical outcome. Clin Infect Dis. 2006;43:1160–1167. doi: 10.1086/508195. [DOI] [PubMed] [Google Scholar]
- 21.Lindgren SW, Samuel JE, Schmitt CK, O'Brien AD. The specific activities of Shiga-like toxin type II (SLT-II) and SLT-II-related toxins of enterohemorrhagic Escherichia coli differ when measured by Vero cell cytotoxicity but not by mouse lethality. Infect Immun. 1994;62:623–631. doi: 10.1128/iai.62.2.623-631.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lindgren SW, Melton AR, O'Brien AD. Virulence of enterohemorrhagic Escherichia coli O91:H21 clinical isolates in an orally infected mouse model. Infect Immun. 1993;61:3832–3842. doi: 10.1128/iai.61.9.3832-3842.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fuller CA, Pellino CA, Flagler MJ, Strasser JE, Weiss AA. Shiga toxin subtypes display dramatic differences in potency. Infect Immun. 2011;79:1329–1337. doi: 10.1128/IAI.01182-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pierard D, Muyldermans G, Moriau L, Stevens D, Lauwers S. Identification of new verocytotoxin type 2 variant B-subunit genes in human and animal Escherichia coli isolates. J Clin Microbiol. 1998;36:3317–3322. doi: 10.1128/jcm.36.11.3317-3322.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stephan R, Hoelzle LE. Characterization of Shiga toxin type 2 variant B-subunit in Escherichia coli strains from asymptomatic human carriers by PCR-RFLP. Lett Appl Microbiol. 2000;31:139–142. doi: 10.1046/j.1365-2672.2000.00778.x. [DOI] [PubMed] [Google Scholar]
- 26.Moxley RA. Edema disease. Vet Clin North Am Food Anim Pract. 2000;16:175–185. doi: 10.1016/s0749-0720(15)30142-0. [DOI] [PubMed] [Google Scholar]
- 27.Hovde CJ, Calderwood SB, Mekalanos JJ, Collier RJ. Evidence that glutamic acid 167 is an active-site residue of Shiga-like toxin I. Proc Natl Acad Sci U S A. 1988;85:2568–2572. doi: 10.1073/pnas.85.8.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fraser ME, Fujinaga M, Cherney MM, Melton-Celsa AR, Twiddy EM, O'Brien AD, James MN. Structure of Shiga toxin type 2 (Stx2) from Escherichia coli O157:H7. J Biol Chem. 2004;279:27511–27517. doi: 10.1074/jbc.M401939200. [DOI] [PubMed] [Google Scholar]
- 29.Melton-Celsa AR, Kokai-Kun JF, O'Brien AD. Activation of Shiga toxin type 2d (Stx2d) by elastase involves cleavage of the C-terminal two amino acids of the A2 peptide in the context of the appropriate B pentamer. Mol Microbiol. 2002;43:207–215. doi: 10.1046/j.1365-2958.2002.02733.x. [DOI] [PubMed] [Google Scholar]
- 30.Stein PE, Boodhoo A, Tyrrell GJ, Brunton JL, Read RJ. Crystal structure of the cell-binding B oligomer of verotoxin-1 from E. coli. . Nature. 1992;355:748–750. doi: 10.1038/355748a0. [DOI] [PubMed] [Google Scholar]
- 31.Fraser ME, Chernaia MM, Kozlov YV, James MN. Crystal structure of the holotoxin from Shigella dysenteriae at 2.5 A resolution. Nat Struct Biol. 1994;1:59–64. doi: 10.1038/nsb0194-59. [DOI] [PubMed] [Google Scholar]
- 32.Fraser ME, Cherney MM, Marcato P, Mulvey GL, Armstrong GD, James MN. Binding of adenine to Stx2, the protein toxin from Escherichia coli O157:H7. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2006;62:627–630. doi: 10.1107/S1744309106021968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Richardson JM, Evans PD, Homans SW, Donohue-Rolfe A. Solution structure of the carbohydrate-binding B-subunit homopentamer of verotoxin VT-1 from E. coli. . Nat Struct Biol. 1997;4:190–193. doi: 10.1038/nsb0397-190. [DOI] [PubMed] [Google Scholar]
- 34.Shimizu H, Field RA, Homans SW, Donohue-Rolfe A. Solution structure of the complex between the B-subunit homopentamer of verotoxin VT-1 from Escherichia coli and the trisaccharide moiety of globotriaosylceramide. Biochemistry. 1998;37:11078–11082. doi: 10.1021/bi980946+. [DOI] [PubMed] [Google Scholar]
- 35.Ling H, Boodhoo A, Hazes B, Cummings MD, Armstrong GD, Brunton JL, Read RJ. Structure of the Shiga-like toxin I B-pentamer complexed with an analogue of its receptor Gb3. Biochemistry. 1998;37:1777–1788. doi: 10.1021/bi971806n. [DOI] [PubMed] [Google Scholar]
- 36.Aletrari MO, McKibbin C, Williams H, Pawar V, Pietroni P, Lord JM, Flitsch SL, Whitehead R, Swanton E, High S, Spooner RA. Eeyarestatin 1 interferes with both retrograde and anterograde intracellular trafficking pathways. PLoS One. 2011;6:e22713. doi: 10.1371/journal.pone.0022713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deresiewicz RL, Calderwood SB, Robertus JD, Collier RJ. Mutations affecting the activity of the Shiga-like toxin I A-chain. Biochemistry. 1992;31:3272–3280. doi: 10.1021/bi00127a032. [DOI] [PubMed] [Google Scholar]
- 38.Di R, Kyu E, Shete V, Saidasan H, Kahn PC, Tumer NE. Identification of amino acids critical for the cytotoxicity of Shiga toxin 1 and 2 in Saccharomyces cerevisiae . Toxicon. 2011;57:525–539. doi: 10.1016/j.toxicon.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.LaPointe P, Wei X, Gariepy J. A role for the protease-sensitive loop region of Shiga-like toxin 1 in the retrotranslocation of its A1 domain from the endoplasmic reticulum lumen. J Biol Chem. 2005;280:23310–23318. doi: 10.1074/jbc.M414193200. [DOI] [PubMed] [Google Scholar]
- 40.McCluskey AJ, Poon GM, Bolewska-Pedyczak E, Srikumar T, Jeram SM, Raught B, Gariepy J. The catalytic subunit of Shiga-like toxin 1 interacts with ribosomal stalk proteins and is inhibited by their conserved C-terminal domain. J Mol Biol. 2008;378:375–386. doi: 10.1016/j.jmb.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 41.McCluskey AJ, Bolewska-Pedyczak E, Jarvik N, Chen G, Sidhu SS, Gariepy J. Charged and hydrophobic surfaces on the a chain of Shiga-like toxin 1 recognize the C-terminal domain of ribosomal stalk proteins. PLoS One. 2012;7:e31191. doi: 10.1371/journal.pone.0031191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lindberg AA, Brown JE, Stromberg N, Westling-Ryd M, Schultz JE, Karlsson KA. Identification of the carbohydrate receptor for Shiga toxin produced by Shigella dysenteriae type 1. J Biol Chem. 1987;262:1779–1785. [PubMed] [Google Scholar]
- 43.Lingwood CA, Law H, Richardson S, Petric M, Brunton JL, De Grandis S, Karmali M. Glycolipid binding of purified and recombinant Escherichia coli produced verotoxin in vitro. J Biol Chem. 1987;262:8834–8839. [PubMed] [Google Scholar]
- 44.Waddell T, Head S, Petric M, Cohen A, Lingwood C. Globotriosyl ceramide is specifically recognized by the Escherichia coli verocytotoxin 2. Biochem Biophys Res Commun. 1988;152:674–679. doi: 10.1016/s0006-291x(88)80091-3. [DOI] [PubMed] [Google Scholar]
- 45.Jacewicz MS, Mobassaleh M, Gross SK, Balasubramanian KA, Daniel PF, Raghavan S, McCluer RH, Keusch GT. Pathogenesis of Shigella diarrhea: XVII. A mammalian cell membrane glycolipid, Gb3, is required but not sufficient to confer sensitivity to Shiga toxin. J Infect Dis. 1994;169:538–546. doi: 10.1093/infdis/169.3.538. [DOI] [PubMed] [Google Scholar]
- 46.Acheson DW, Moore R, De Breucker S, Lincicome L, Jacewicz M, Skutelsky E, Keusch GT. Translocation of Shiga toxin across polarized intestinal cells in tissue culture. Infect Immun. 1996;64:3294–3300. doi: 10.1128/iai.64.8.3294-3300.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Okuda T, Tokuda N, Numata S, Ito M, Ohta M, Kawamura K, Wiels J, Urano T, Tajima O, Furukawa K. Targeted disruption of Gb3/CD77 synthase gene resulted in the complete deletion of globo-series glycosphingolipids and loss of sensitivity to verotoxins. J Biol Chem. 2006;281:10230–10235. doi: 10.1074/jbc.M600057200. [DOI] [PubMed] [Google Scholar]
- 48.Tarabuso AL. Fabry disease. Skinmed. 2011;9:173–177. [PubMed] [Google Scholar]
- 49.Cilmi SA, Karalius BJ, Choy W, Smith RN, Butterton JR. Fabry disease in mice protects against lethal disease caused by Shiga toxin-expressing enterohemorrhagic Escherichia coli . J Infect Dis. 2006;194:1135–1140. doi: 10.1086/507705. [DOI] [PubMed] [Google Scholar]
- 50.Nakajima H, Kiyokawa N, Katagiri YU, Taguchi T, Suzuki T, Sekino T, Mimori K, Ebata T, Saito M, Nakao H, Takeda T, Fujimoto J. Kinetic analysis of binding between Shiga toxin and receptor glycolipid Gb3Cer by surface plasmon resonance. J Biol Chem. 2001;276:42915–42922. doi: 10.1074/jbc.M106015200. [DOI] [PubMed] [Google Scholar]
- 51.DeGrandis S, Law H, Brunton J, Gyles C, Lingwood CA. Globotetraosylceramide is recognized by the pig edema disease toxin. J Biol Chem. 1989;264:12520–12525. [PubMed] [Google Scholar]
- 52.Samuel JE, Perera LP, Ward S, O'Brien AD, Ginsburg V, Krivan HC. Comparison of the glycolipid receptor specificities of Shiga-like toxin type II and Shiga-like toxin type II variants. Infect Immun. 1990;58:611–618. doi: 10.1128/iai.58.3.611-618.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Devenish J, Gyles C, LaMarre J. Binding of Escherichia coli verotoxins to cell surface protein on wild-type and globotriaosylceramide-deficient Vero cells. Can J Microbiol. 1998;44:28–34. [PubMed] [Google Scholar]
- 54.Katagiri YU, Mori T, Nakajima H, Katagiri C, Taguchi T, Takeda T, Kiyokawa N, Fujimoto J. Activation of Src family kinase yes induced by Shiga toxin binding to globotriaosyl ceramide (Gb3/CD77) in low density, detergent-insoluble microdomains. J Biol Chem. 1999;274:35278–35282. doi: 10.1074/jbc.274.49.35278. [DOI] [PubMed] [Google Scholar]
- 55.Kovbasnjuk O, Edidin M, Donowitz M. Role of lipid rafts in Shiga toxin 1 interaction with the apical surface of Caco-2 cells. J Cell Sci. 2001;114:4025–4031. doi: 10.1242/jcs.114.22.4025. [DOI] [PubMed] [Google Scholar]
- 56.Falguieres T, Romer W, Amessou M, Afonso C, Wolf C, Tabet JC, Lamaze C, Johannes L. Functionally different pools of Shiga toxin receptor, globotriaosyl ceramide, in HeLa cells. FEBS J. 2006;273:5205–5218. doi: 10.1111/j.1742-4658.2006.05516.x. [DOI] [PubMed] [Google Scholar]
- 57.Kiarash A, Boyd B, Lingwood CA. Glycosphingolipid receptor function is modified by fatty acid content. Verotoxin 1 and verotoxin 2c preferentially recognize different globotriaosyl ceramide fatty acid homologues. J Biol Chem. 1994;269:11138–11146. [PubMed] [Google Scholar]
- 58.Pellizzari A, Pang H, Lingwood CA. Binding of verocytotoxin 1 to its receptor is influenced by differences in receptor fatty acid content. Biochemistry. 1992;31:1363–1370. doi: 10.1021/bi00120a011. [DOI] [PubMed] [Google Scholar]
- 59.Khan F, Proulx F, Lingwood CA. Detergent-resistant globotriaosyl ceramide may define verotoxin/glomeruli-restricted hemolytic uremic syndrome pathology. Kidney Int. 2009;75:1209–1216. doi: 10.1038/ki.2009.7. [DOI] [PubMed] [Google Scholar]
- 60.Nyholm PG, Magnusson G, Zheng Z, Norel R, Binnington-Boyd B, Lingwood CA. Two distinct binding sites for globotriaosyl ceramide on verotoxins: identification by molecular modelling and confirmation using deoxy analogues and a new glycolipid receptor for all verotoxins. Chem Biol. 1996;3:263–275. doi: 10.1016/s1074-5521(96)90106-4. [DOI] [PubMed] [Google Scholar]
- 61.Thompson GS, Shimizu H, Homans SW, Donohue-Rolfe A. Localization of the binding site for the oligosaccharide moiety of Gb3 on verotoxin 1 using NMR residual dipolar coupling measurements. Biochemistry. 2000;39:13153–13156. doi: 10.1021/bi001394+. [DOI] [PubMed] [Google Scholar]
- 62.Bast DJ, Banerjee L, Clark C, Read RJ, Brunton JL. The identification of three biologically relevant globotriaosyl ceramide receptor binding sites on the Verotoxin 1 B subunit. Mol Microbiol. 1999;32:953–960. doi: 10.1046/j.1365-2958.1999.01405.x. [DOI] [PubMed] [Google Scholar]
- 63.Soltyk AM, MacKenzie CR, Wolski VM, Hirama T, Kitov PI, Bundle DR, Brunton JL. A mutational analysis of the globotriaosylceramide-binding sites of verotoxin VT1. J Biol Chem. 2002;277:5351–5359. doi: 10.1074/jbc.M107472200. [DOI] [PubMed] [Google Scholar]
- 64.Kitova EN, Kitov PI, Paszkiewicz E, Kim J, Mulvey GL, Armstrong GD, Bundle DR, Klassen JS. Affinities of Shiga toxins 1 and 2 for univalent and oligovalent Pk-trisaccharide analogs measured by electrospray ionization mass spectrometry. Glycobiology. 2007;17:1127–1137. doi: 10.1093/glycob/cwm081. [DOI] [PubMed] [Google Scholar]
- 65.Nishikawa K, Matsuoka K, Watanabe M, Igai K, Hino K, Hatano K, Yamada A, Abe N, Terunuma D, Kuzuhara H, Natori Y. Identification of the optimal structure required for a Shiga toxin neutralizer with oriented carbohydrates to function in the circulation. J Infect Dis. 2005;191:2097–2105. doi: 10.1086/430388. [DOI] [PubMed] [Google Scholar]
- 66.Watanabe M, Igai K, Matsuoka K, Miyagawa A, Watanabe T, Yanoshita R, Samejima Y, Terunuma D, Natori Y, Nishikawa K. Structural analysis of the interaction between Shiga toxin B subunits and linear polymers bearing clustered globotriose residues. Infect Immun. 2006;74:1984–1988. doi: 10.1128/IAI.74.3.1984-1988.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Holgersson J, Jovall PA, Breimer ME. Glycosphingolipids of human large intestine: detailed structural characterization with special reference to blood group compounds and bacterial receptor structures. J Biochem. 1991;110:120–131. doi: 10.1093/oxfordjournals.jbchem.a123530. [DOI] [PubMed] [Google Scholar]
- 68.Malyukova I, Murray KF, Zhu C, Boedeker E, Kane A, Patterson K, Peterson JR, Donowitz M, Kovbasnjuk O. Macropinocytosis in Shiga toxin 1 uptake by human intestinal epithelial cells and transcellular transcytosis. Am J Physiol Gastrointest Liver Physiol. 2009;296:G78–G92. doi: 10.1152/ajpgi.90347.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hurley BP, Thorpe CM, Acheson DW. Shiga toxin translocation across intestinal epithelial cells is enhanced by neutrophil transmigration. Infect Immun. 2001;69:6148–6155. doi: 10.1128/IAI.69.10.6148-6155.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Philpott DJ, Ackerley CA, Kiliaan AJ, Karmali MA, Perdue MH, Sherman PM. Translocation of verotoxin-1 across T84 monolayers: mechanism of bacterial toxin penetration of epithelium. Am J Physiol. 1997;273:G1349–G1358. doi: 10.1152/ajpgi.1997.273.6.G1349. [DOI] [PubMed] [Google Scholar]
- 71.Schuller S, Frankel G, Phillips AD. Interaction of Shiga toxin from Escherichia coli with human intestinal epithelial cell lines and explants: Stx2 induces epithelial damage in organ culture. Cell Microbiol. 2004;6:289–301. doi: 10.1046/j.1462-5822.2004.00370.x. [DOI] [PubMed] [Google Scholar]
- 72.Zumbrun SD, Hanson L, Sinclair JF, Freedy J, Melton-Celsa AR, Rodriguez-Canales J, Hanson JC, O'Brien AD. Human intestinal tissue and cultured colonic cells contain globotriaosylceramide synthase mRNA and the alternate Shiga toxin receptor globotetraosylceramide. Infect Immun. 2010;78:4488–4499. doi: 10.1128/IAI.00620-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jacewicz MS, Acheson DW, Mobassaleh M, Donohue-Rolfe A, Balasubramanian KA, Keusch GT. Maturational regulation of globotriaosylceramide, the Shiga-like toxin 1 receptor, in cultured human gut epithelial cells. J Clin Invest. 1995;96:1328–1335. doi: 10.1172/JCI118168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zumbrun SD, Melton-Celsa AR, Smith MA, Gilbreath JJ, Merrell DS, O'Brien AD. Dietary choice affects Shiga toxin-producing Escherichia coli (STEC) O157:H7 colonization and disease. Proc Natl Acad Sci U S A. 2013;110:E2126–E2133. doi: 10.1073/pnas.1222014110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schuller S, Heuschkel R, Torrente F, Kaper JB, Phillips AD. Shiga toxin binding in normal and inflamed human intestinal mucosa. Microbes Infect. 2007;9:35–39. doi: 10.1016/j.micinf.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 76.Bergan J, Dyve Lingelem AB, Simm R, Skotland T, Sandvig K. Shiga toxins. Toxicon. 2012;60:1085–1107. doi: 10.1016/j.toxicon.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 77.Torgersen ML, Lauvrak SU, Sandvig K. The A-subunit of surface-bound Shiga toxin stimulates clathrin-dependent uptake of the toxin. FEBS J. 2005;272:4103–4113. doi: 10.1111/j.1742-4658.2005.04835.x. [DOI] [PubMed] [Google Scholar]
- 78.Romer W, Berland L, Chambon V, Gaus K, Windschiegl B, Tenza D, Aly MR, Fraisier V, Florent JC, Perrais D, Lamaze C, Raposo G, Steinem C, Sens P, Bassereau P, Johannes L. Shiga toxin induces tubular membrane invaginations for its uptake into cells. Nature. 2007;450:670–675. doi: 10.1038/nature05996. [DOI] [PubMed] [Google Scholar]
- 79.Sandvig K, Garred O, Prydz K, Kozlov JV, Hansen SH, van Deurs B. Retrograde transport of endocytosed Shiga toxin to the endoplasmic reticulum. Nature. 1992;358:510–512. doi: 10.1038/358510a0. [DOI] [PubMed] [Google Scholar]
- 80.Sandvig K, Bergan J, Dyve AB, Skotland T, Torgersen ML. Endocytosis and retrograde transport of Shiga toxin. Toxicon. 2010;56:1181–1185. doi: 10.1016/j.toxicon.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 81.Garred O, van Deurs B, Sandvig K. Furin-induced cleavage and activation of Shiga toxin. J Biol Chem. 1995;270:10817–10821. doi: 10.1074/jbc.270.18.10817. [DOI] [PubMed] [Google Scholar]
- 82.Raa H, Grimmer S, Schwudke D, Bergan J, Walchli S, Skotland T, Shevchenko A, Sandvig K. Glycosphingolipid requirements for endosome-to-Golgi transport of Shiga toxin. Traffic. 2009;10:868–882. doi: 10.1111/j.1600-0854.2009.00919.x. [DOI] [PubMed] [Google Scholar]
- 83.Sandvig K, Skotland T, van Deurs B, Klokk TI. Retrograde transport of protein toxins through the Golgi apparatus. Histochem Cell Biol. 2013 doi: 10.1007/s00418-013-1111-z. [DOI] [PubMed] [Google Scholar]
- 84.Spooner RA, Lord JM. How ricin and Shiga toxin reach the cytosol of target cells: retrotranslocation from the endoplasmic reticulum. Curr Top Microbiol Immunol. 2012;357:19–40. doi: 10.1007/82_2011_154. [DOI] [PubMed] [Google Scholar]
- 85.Tam PJ, Lingwood CA. Membrane cytosolic translocation of verotoxin A1 subunit in target cells. Microbiology. 2007;153:2700–2710. doi: 10.1099/mic.0.2007/006858-0. [DOI] [PubMed] [Google Scholar]
- 86.Obrig TG, Moran TP, Brown JE. The mode of action of Shiga toxin on peptide elongation of eukaryotic protein synthesis. Biochem J. 1987;244:287–294. doi: 10.1042/bj2440287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Furutani M, Kashiwagi K, Ito K, Endo Y, Igarashi K. Comparison of the modes of action of a Vero toxin (a Shiga-like toxin) from Escherichia coli, of ricin, and of alpha-sarcin. Arch Biochem Biophys. 1992;293:140–146. doi: 10.1016/0003-9861(92)90376-8. [DOI] [PubMed] [Google Scholar]
- 88.Warnier M, Romer W, Geelen J, Lesieur J, Amessou M, van den Heuvel L, Monnens L, Johannes L. Trafficking of Shiga toxin/Shiga-like toxin-1 in human glomerular microvascular endothelial cells and human mesangial cells. Kidney Int. 2006;70:2085–2091. doi: 10.1038/sj.ki.5001989. [DOI] [PubMed] [Google Scholar]
- 89.Jandhyala DM, Thorpe CM, Magun B. Ricin and Shiga toxins: effects on host cell signal transduction. Curr Top Microbiol Immunol. 2012;357:41–65. doi: 10.1007/82_2011_181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lee MS, Kim MH, Tesh VL. Shiga toxins expressed by human pathogenic bacteria induce immune responses in host cells. J Microbiol. 2013;51:724–730. doi: 10.1007/s12275-013-3429-6. [DOI] [PubMed] [Google Scholar]
- 91.Tesh VL. The induction of apoptosis by Shiga toxins and ricin. Curr Top Microbiol Immunol. 2012;357:137–178. doi: 10.1007/82_2011_155. [DOI] [PubMed] [Google Scholar]
- 92.Pai CH, Kelly JK, Meyers GL. Experimental infection of infant rabbits with verotoxin-producing Escherichia coli . Infect Immun. 1986;51:16–23. doi: 10.1128/iai.51.1.16-23.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Keenan KP, Sharpnack DD, Collins H, Formal SB, O'Brien AD. Morphologic evaluation of the effects of Shiga toxin and E. coli Shiga-like toxin on the rabbit intestine. Am J Pathol. 1986;125:69–80. [PMC free article] [PubMed] [Google Scholar]
- 94.Tesh VL, Burris JA, Owens JW, Gordon VM, Wadolkowski EA, O'Brien AD, Samuel JE. Comparison of the relative toxicities of Shiga-like toxins type I and type II for mice. Infect Immun. 1993;61:3392–3402. doi: 10.1128/iai.61.8.3392-3402.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Smith MJ, Teel LD, Carvalho HM, Melton-Celsa AR, O'Brien AD. Development of a hybrid Shiga holotoxoid vaccine to elicit heterologous protection against Shiga toxins types 1 and 2. Vaccine. 2006;24:4122–4129. doi: 10.1016/j.vaccine.2006.02.035. [DOI] [PubMed] [Google Scholar]
- 96.Soborg B, Lassen SG, Muller L, Jensen T, Ethelberg S, Molbak K, Scheutz F. A verocytotoxin-producing E. coli outbreak with a surprisingly high risk of haemolytic uraemic syndrome, Denmark, September-October 2012. Euro Surveill. 2013;18 [PubMed] [Google Scholar]
- 97.Boerlin P, McEwen SA, Boerlin-Petzold F, Wilson JB, Johnson RP, Gyles CL. Associations between virulence factors of Shiga toxin-producing Escherichia coli and disease in humans. J Clin Microbiol. 1999;37:497–503. doi: 10.1128/jcm.37.3.497-503.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ostroff SM, Tarr PI, Neill MA, Lewis JH, Hargrett-Bean N, Kobayashi JM. Toxin genotypes and plasmid profiles as determinants of systemic sequelae in Escherichia coli O157:H7 infections. J Infect Dis. 1989;160:994–998. doi: 10.1093/infdis/160.6.994. [DOI] [PubMed] [Google Scholar]
- 99.Russo LM, Melton-Celsa AR, Smith MA, Smith MJ, O'Brien A D. Oral intoxication of mice with Shiga toxin type 2a (Stx2a) and protection by anti-Stx2a monoclonal antibody 11E10. Infect Immun. 2013 doi: 10.1128/IAI.01264-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Louise CB, Obrig TG. Specific interaction of Escherichia coli O157:H7-derived Shiga-like toxin II with human renal endothelial cells. J Infect Dis. 1995;172:1397–1401. doi: 10.1093/infdis/172.5.1397. [DOI] [PubMed] [Google Scholar]
- 101.Chark D, Nutikka A, Trusevych N, Kuzmina J, Lingwood C. Differential carbohydrate epitope recognition of globotriaosyl ceramide by verotoxins and a monoclonal antibody. Eur J Biochem. 2004;271:405–417. doi: 10.1046/j.1432-1033.2003.03941.x. [DOI] [PubMed] [Google Scholar]
- 102.Head SC, Karmali MA, Lingwood CA. Preparation of VT1 and VT2 hybrid toxins from their purified dissociated subunits. Evidence for B subunit modulation of a subunit function. J Biol Chem. 1991;266:3617–3621. [PubMed] [Google Scholar]
- 103.Gallegos KM, Conrady DG, Karve SS, Gunasekera TS, Herr AB, Weiss AA. Shiga toxin binding to glycolipids and glycans. PLoS One. 2012;7:e30368. doi: 10.1371/journal.pone.0030368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mahfoud R, Manis A, Binnington B, Ackerley C, Lingwood CA. A major fraction of glycosphingolipids in model and cellular cholesterol-containing membranes is undetectable by their binding proteins. J Biol Chem. 2010;285:36049–36059. doi: 10.1074/jbc.M110.110189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gamage SD, McGannon CM, Weiss AA. Escherichia coli serogroup O107/O117 lipopolysaccharide binds and neutralizes Shiga toxin 2. J Bacteriol. 2004;186:5506–5512. doi: 10.1128/JB.186.16.5506-5512.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kimura T, Tani S, Matsumoto Yi Y, Takeda T. Serum amyloid P component is the Shiga toxin 2-neutralizing factor in human blood. J Biol Chem. 2001;276:41576–41579. doi: 10.1074/jbc.M107819200. [DOI] [PubMed] [Google Scholar]
- 107.Conrady DG, Flagler MJ, Friedmann DR, Vander Wielen BD, Kovall RA, Weiss AA, Herr AB. Molecular basis of differential B-pentamer stability of Shiga toxins 1 and 2. PLoS One. 2010;5:e15153. doi: 10.1371/journal.pone.0015153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tam P, Mahfoud R, Nutikka A, Khine AA, Binnington B, Paroutis P, Lingwood C. Differential intracellular transport and binding of verotoxin 1 and verotoxin 2 to globotriaosylceramide-containing lipid assemblies. J Cell Physiol. 2008;216:750–763. doi: 10.1002/jcp.21456. [DOI] [PubMed] [Google Scholar]
- 109.Matussek A, Lauber J, Bergau A, Hansen W, Rohde M, Dittmar KE, Gunzer M, Mengel M, Gatzlaff P, Hartmann M, Buer J, Gunzer F. Molecular and functional analysis of Shiga toxin-induced response patterns in human vascular endothelial cells. Blood. 2003;102:1323–1332. doi: 10.1182/blood-2002-10-3301. [DOI] [PubMed] [Google Scholar]
- 110.Lentz EK, Leyva-Illades D, Lee MS, Cherla RP, Tesh VL. Differential response of the human renal proximal tubular epithelial cell line HK-2 to Shiga toxin types 1 and 2. Infect Immun. 2011;79:3527–3540. doi: 10.1128/IAI.05139-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Stearns-Kurosawa DJ, Collins V, Freeman S, Tesh VL, Kurosawa S. Distinct physiologic and inflammatory responses elicited in baboons after challenge with Shiga toxin type 1 or 2 from enterohemorrhagic Escherichia coli . Infect Immun. 2010;78:2497–2504. doi: 10.1128/IAI.01435-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bu F, Borsa N, Gianluigi A, Smith RJ. Familial atypical hemolytic uremic syndrome: a review of its genetic and clinical aspects. Clin Dev Immunol. 2012;2012:370–426. doi: 10.1155/2012/370426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Riley LW, Remis RS, Helgerson SD, McGee HB, Wells JG, Davis BR, Hebert RJ, Olcott ES, Johnson LM, Hargrett NT, Blake PA, Cohen ML. Hemorrhagic colitis associated with a rare Escherichia coli serotype. N Engl J Med. 1983;308:681–685. doi: 10.1056/NEJM198303243081203. [DOI] [PubMed] [Google Scholar]
- 114.Frank C, Werber D, Cramer JP, Askar M, Faber M, an der Heiden M, Bernard H, Fruth A, Prager R, Spode A, Wadl M, Zoufaly A, Jordan S, Kemper MJ, Follin P, Muller L, King LA, Rosner B, Buchholz U, Stark K, Krause G. Epidemic profile of Shiga-toxin-producing Escherichia coli O104:H4 outbreak in Germany. N Engl J Med. 2011;365:1771–1780. doi: 10.1056/NEJMoa1106483. [DOI] [PubMed] [Google Scholar]
- 115.Sauter KA, Melton-Celsa AR, Larkin K, Troxell ML, O'Brien AD, Magun BE. Mouse model of hemolytic-uremic syndrome caused by endotoxin-free Shiga toxin 2 (Stx2) and protection from lethal outcome by anti-Stx2 antibody. Infect Immun. 2008;76:4469–4478. doi: 10.1128/IAI.00592-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fontaine A, Arondel J, Sansonetti PJ. Role of Shiga toxin in the pathogenesis of bacillary dysentery, studied by using a Tox- mutant of Shigella dysenteriae 1. Infect Immun. 1988;56:3099–3109. doi: 10.1128/iai.56.12.3099-3109.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chaisri U, Nagata M, Kurazono H, Horie H, Tongtawe P, Hayashi H, Watanabe T, Tapchaisri P, Chongsa-nguan M, Chaicumpa W. Localization of Shiga toxins of enterohaemorrhagic Escherichia coli in kidneys of paediatric and geriatric patients with fatal haemolytic uraemic syndrome. Microb Pathog. 2001;31:59–67. doi: 10.1006/mpat.2001.0447. [DOI] [PubMed] [Google Scholar]
- 118.Uchida H, Kiyokawa N, Horie H, Fujimoto J, Takeda T. The detection of Shiga toxins in the kidney of a patient with hemolytic uremic syndrome. Pediatr Res. 1999;45:133–137. doi: 10.1203/00006450-199901000-00022. [DOI] [PubMed] [Google Scholar]
- 119.Tazzari PL, Ricci F, Carnicelli D, Caprioli A, Tozzi AE, Rizzoni G, Conte R, Brigotti M. Flow cytometry detection of Shiga toxins in the blood from children with hemolytic uremic syndrome. Cytometry B Clin Cytom. 2004;61:40–44. doi: 10.1002/cyto.b.20022. [DOI] [PubMed] [Google Scholar]
- 120.Bitzan M, Poole R, Mehran M, Sicard E, Brockus C, Thuning-Roberson C, Riviere M. Safety and pharmacokinetics of chimeric anti-Shiga toxin 1 and anti-Shiga toxin 2 monoclonal antibodies in healthy volunteers. Antimicrob Agents Chemother. 2009;53:3081–3087. doi: 10.1128/AAC.01661-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dowling TC, Chavaillaz PA, Young DG, Melton-Celsa A, O'Brien A, Thuning-Roberson C, Edelman R, Tacket CO. Phase 1 safety and pharmacokinetic study of chimeric murine-human monoclonal antibody c alpha Stx2 administered intravenously to healthy adult volunteers. Antimicrob Agents Chemother. 2005;49:1808–1812. doi: 10.1128/AAC.49.5.1808-1812.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.McKee ML, Melton-Celsa AR, Moxley RA, Francis DH, O'Brien AD. Enterohemorrhagic Escherichia coli O157:H7 requires intimin to colonize the gnotobiotic pig intestine and to adhere to HEp-2 cells. Infect Immun. 1995;63:3739–3744. doi: 10.1128/iai.63.9.3739-3744.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Judge NA, Mason HS, O'Brien AD. Plant cell-based intimin vaccine given orally to mice primed with intimin reduces time of Escherichia coli O157:H7 shedding in feces. Infect Immun. 2004;72:168–175. doi: 10.1128/IAI.72.1.168-175.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Girardeau JP, Dalmasso A, Bertin Y, Ducrot C, Bord S, Livrelli V, Vernozy-Rozand C, Martin C. Association of virulence genotype with phylogenetic background in comparison to different seropathotypes of Shiga toxin-producing Escherichia coli isolates. J Clin Microbiol. 2005;43:6098–6107. doi: 10.1128/JCM.43.12.6098-6107.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zumbrun SD, Melton-Celsa AR, O'Brien AD. When a healthy diet turns deadly. Gut Microbes. 2013;5 doi: 10.4161/gmic.26263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Petruzziello-Pellegrini TN, Moslemi-Naeini M, Marsden PA. New insights into Shiga toxin-mediated endothelial dysfunction in hemolytic uremic syndrome. Virulence. 2013;4:556–563. doi: 10.4161/viru.26143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Keir LS, Saleem MA. Current evidence for the role of complement in the pathogenesis of Shiga toxin haemolytic uraemic syndrome. Pediatr Nephrol. 2013 doi: 10.1007/s00467-013-2561-1. [DOI] [PubMed] [Google Scholar]
- 128.Zoja C, Buelli S, Morigi M. Shiga toxin-associated hemolytic uremic syndrome: pathophysiology of endothelial dysfunction. Pediatr Nephrol. 2010;25:2231–2240. doi: 10.1007/s00467-010-1522-1. [DOI] [PubMed] [Google Scholar]
- 129.Andreoli SP, Trachtman H, Acheson DW, Siegler RL, Obrig TG. Hemolytic uremic syndrome: epidemiology, pathophysiology, and therapy. Pediatr Nephrol. 2002;17:293–298. doi: 10.1007/s00467-001-0783-0. [DOI] [PubMed] [Google Scholar]
- 130.Mayer CL, Leibowitz CS, Kurosawa S, Stearns-Kurosawa DJ. Shiga toxins and the pathophysiology of hemolytic uremic syndrome in humans and animals. Toxins (Basel) 2012;4:1261–1287. doi: 10.3390/toxins4111261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Stahl AL, Sartz L, Karpman D. Complement activation on platelet-leukocyte complexes and microparticles in enterohemorrhagic Escherichia coli-induced hemolytic uremic syndrome. Blood. 2011;117:5503–5513. doi: 10.1182/blood-2010-09-309161. [DOI] [PubMed] [Google Scholar]
- 132.Thurman JM, Marians R, Emlen W, Wood S, Smith C, Akana H, Holers VM, Lesser M, Kline M, Hoffman C, Christen E, Trachtman H. Alternative pathway of complement in children with diarrhea-associated hemolytic uremic syndrome. Clin J Am Soc Nephrol. 2009;4:1920–1924. doi: 10.2215/CJN.02730409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hale TL, Formal SB. Cytotoxicity of Shigella dysenteriae 1 for cultured mammalian cells. Am J Clin Nutr. 1980;33:2485–2490. doi: 10.1093/ajcn/33.11.2485. [DOI] [PubMed] [Google Scholar]
- 134.Koch C, Hertwig S, Lurz R, Appel B, Beutin L. Isolation of a lysogenic bacteriophage carrying the stx(1(OX3)) gene, which is closely associated with Shiga toxin-producing Escherichia coli strains from sheep and humans. J Clin Microbiol. 2001;39:3992–3998. doi: 10.1128/JCM.39.11.3992-3998.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Paton AW, Beutin L, Paton JC. Heterogeneity of the amino-acid sequences of Escherichia coli Shiga-like toxin type-I operons. Gene. 1995;153:71–74. doi: 10.1016/0378-1119(94)00777-p. [DOI] [PubMed] [Google Scholar]
- 136.Burk C, Dietrich R, Acar G, Moravek M, Bulte M, Martlbauer E. Identification and characterization of a new variant of Shiga toxin 1 in Escherichia coli ONT:H19 of bovine origin. J Clin Microbiol. 2003;41:2106–2112. doi: 10.1128/JCM.41.5.2106-2112.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Paton AW, Paton JC, Manning PA. Polymerase chain reaction amplification, cloning and sequencing of variant Escherichia coli Shiga-like toxin type II operons. Microb Pathog. 1993;15:77–82. doi: 10.1006/mpat.1993.1058. [DOI] [PubMed] [Google Scholar]
- 138.Persson S, Olsen KE, Ethelberg S, Scheutz F. Subtyping method for Escherichia coli Shiga toxin (verocytotoxin) 2 variants and correlations to clinical manifestations. J Clin Microbiol. 2007;45:2020–2024. doi: 10.1128/JCM.02591-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Weinstein DL, Jackson MP, Samuel JE, Holmes RK, O'Brien AD. Cloning and sequencing of a Shiga-like toxin type II variant from Escherichia coli strain responsible for edema disease of swine. J Bacteriol. 1988;170:4223–4230. doi: 10.1128/jb.170.9.4223-4230.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Schmidt H, Scheef J, Morabito S, Caprioli A, Wieler LH, Karch H. A new Shiga toxin 2 variant (Stx2f) from Escherichia coli isolated from pigeons. Appl Environ Microbiol. 2000;66:1205–1208. doi: 10.1128/aem.66.3.1205-1208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Leung PH, Peiris JS, Ng WW, Robins-Browne RM, Bettelheim KA, Yam WC. A newly discovered verotoxin variant, VT2g, produced by bovine verocytotoxigenic Escherichia coli . Appl Environ Microbiol. 2003;69:7549–7553. doi: 10.1128/AEM.69.12.7549-7553.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]