Table 1. Optimization and scope of copper-catalyzed direct hydroamination of aryl alkynes.
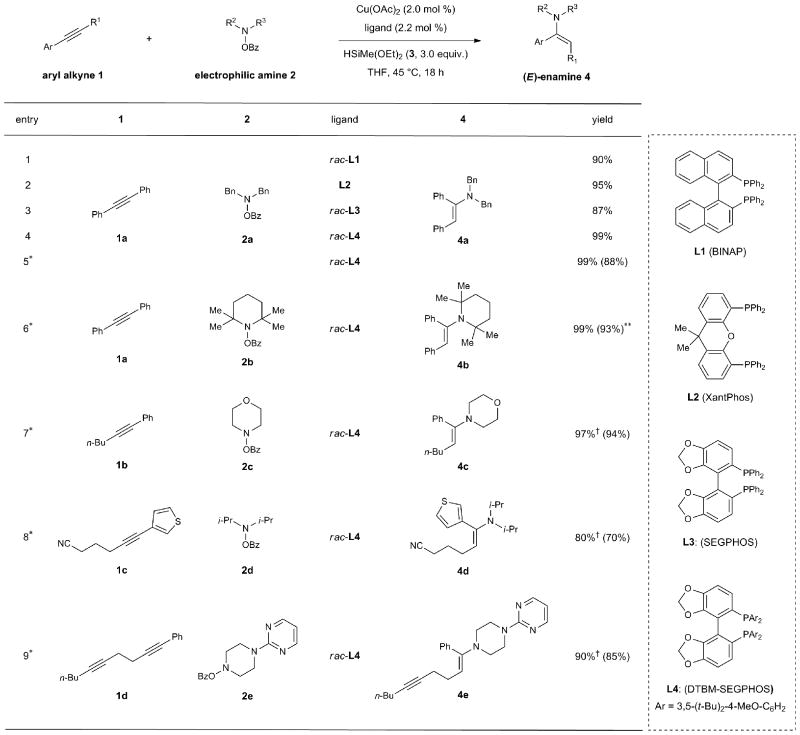
|
Conditions: 1a (0.2 mmol), 2a (0.24 mmol), 3 (0.6 mmol), Cu(OAc)2 (2.0 mol %), ligand (2.2 mol %), THF (1 M), 45 °C, 18 h. Yields and stereoselectivities were determined by 1H NMR analysis using 1,1,2,2-tetracholoroethane as an internal standard. In all cases only (E)-enamine products were observed.
Conditions: 1 (1.0 mmol), 2 (1.2 mmol), 3 (3.0 mmol), Cu(OAc)2 (2.0 mol %), rac-L4 (2.2 mol %), THF (1 M), 45 °C, 18 h. Isolated yields of products after reduction are given in parentheses (average of two runs).
Isolated yield of enamine after purification by flash column chromatography.
Major regioisomers are shown; in all cases, <5% of the minor regioisomer was observed as determined by 1H NMR analysis of the crude enamine product.
