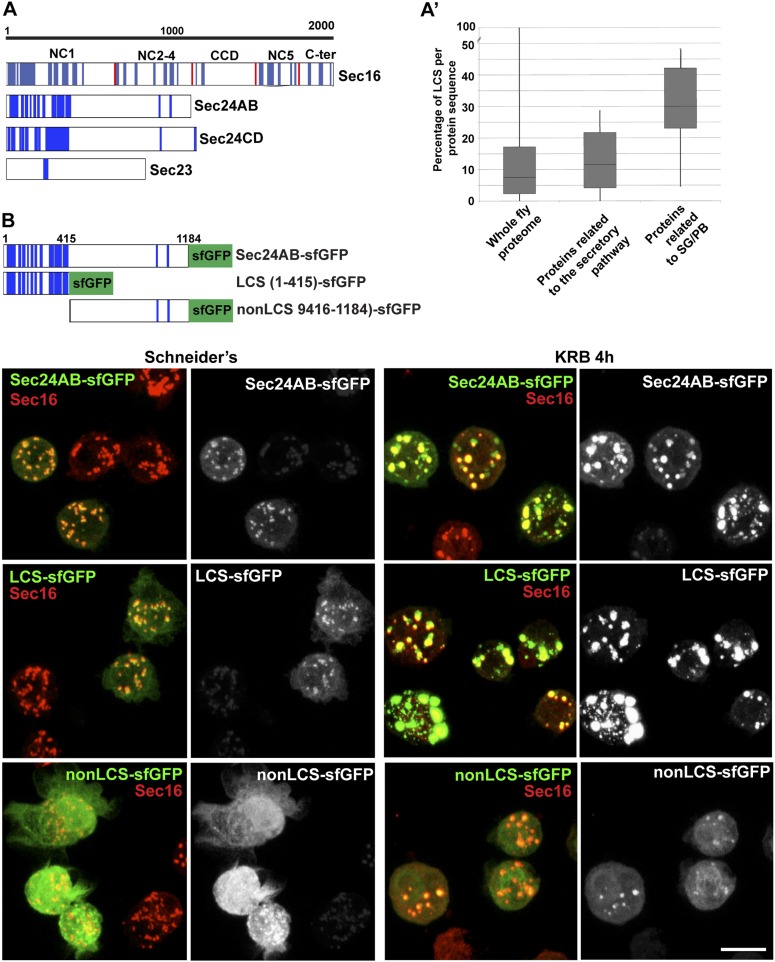
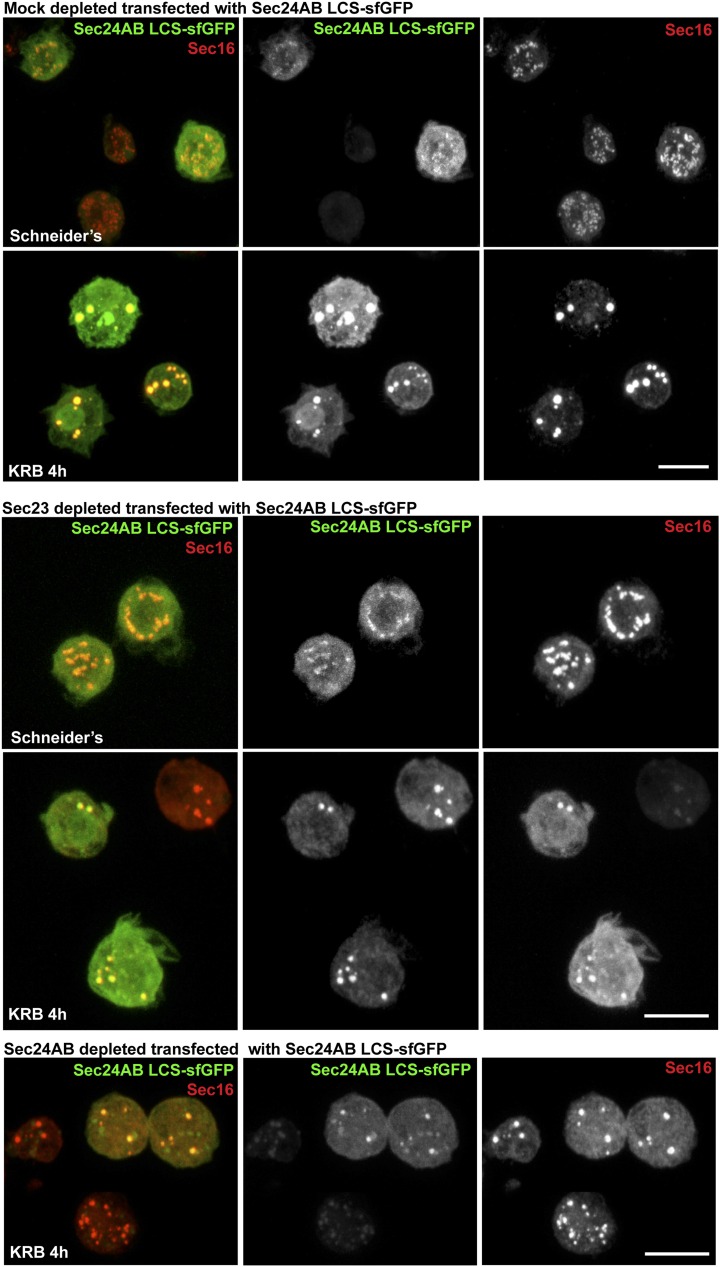
Figure 6. Sec body proteins contain low complexity sequences that are necessary for Sec body recruitment.
(A–A′) Schematic representation of the Low Complexity Sequences (blue bars) in Sec16, Sec24AB, Sec24CD, and Sec23 (A). The red bars mark the boundaries of the Sec16 domains. Genome wide analysis of Low Complexity Sequence (LCS) in the Drosophila proteome, in proteins related to the secretory pathway and proteins related to Stress Granules/P-bodies (A′). B: IF localization of sfGFP-tagged full-length Sec24B, Sec24AB LCS, and Sec24AB nonLCS in S2 cells in Schneider's and KRB for 4 hr, together with endogenous Sec16 (red). Scale bars: 10 μm.
DOI: http://dx.doi.org/10.7554/eLife.04132.015