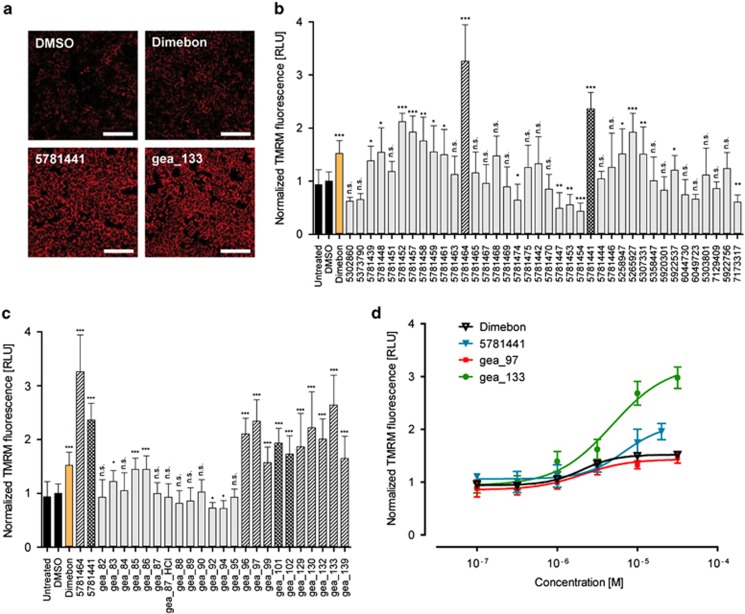
Figure 3.
The effect of tetrahydrocarbazole analogs on mitochondrial membrane potential. (a) Representative TMRM-staining images of HEK293 cells pretreated for 1 h with the indicated tetrahydrocarbazoles (10 μM) or Dimebon (10 μM) as a positive control, relative to DMSO-treated control (scale bar, 100 μm). (b) Quantification of the average TMRM-staining signals showing relative intensity for commercially available analogs of the tetrahydrocarbazole lead structure upon 1 h pretreatment of HEK293 cells (10 μM). The bars highlighted with single- and double-stripes represent the most active compounds 5781464 and 5781441, which, respectively, possess N-(1-benzylpiperidin-4-yl) and N-(1-phenethylpiperidin-4-yl) groups at their R2 position. (c) Quantification of average TMRM intensity for synthesized tetrahydrocarbazole derivatives tested at 10 μM. The marked single-striped bars represent the analogous structures similar to 5781464, possessing N-(1-benzylpiperidin-4-yl) at their R2 position, and double-striped bars represent derivative compounds similar to 5781441, which contain N-(1-phenethylpiperidin-4-yl) group at the R2 position. (d) Quantification of average dose-dependent TMRM relative intensities for three select tetrahydrocarbazoles tested at six different concentrations relative to Dimebon. The EC50 of all analogs tested lies at low micromolar range. All the values are normalized to DMSO value, which is set to 1. (n.s., non-significant; *P<0.05, **P<0.01 and ***P<0.001; n=8). DMSO, dimethyl sulfoxide; TMRM, tetramethylrhodamine methyl ester.