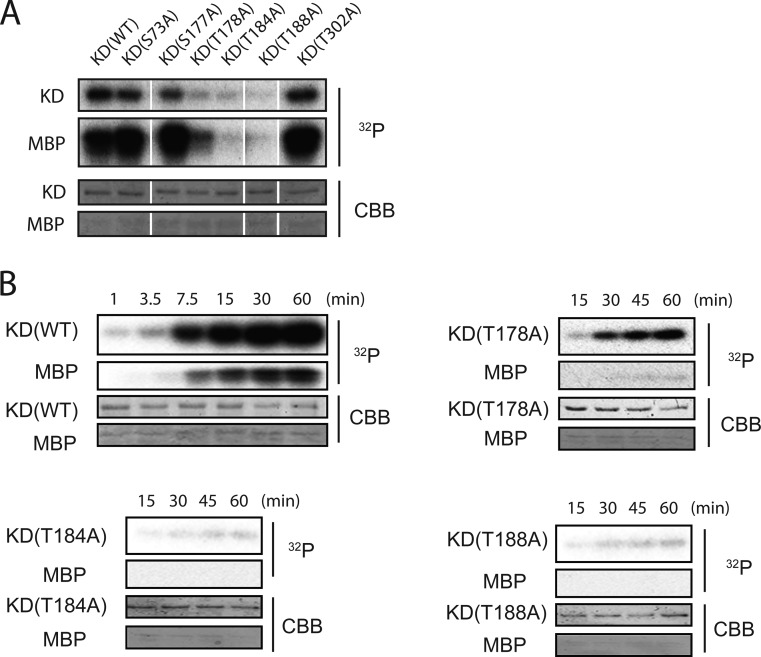
Figure 2.
Effect of mutation of each phosphorylation site on protein kinase activity of hMyo3A-KD. (A) Effect of mutation at the phosphorylation sites on the protein kinase activity and autophosphorylation of hMyo3A-KD. Upper, phosphoimages; lower, input amount of hMyo3A-KD mutants and myelin basic protein (MBP) stained with Coomassie Brilliant Blue R-250. hMyo3A-KD mutants (1 μg) were incubated with 0.25 mM [γ-32P]ATP and myelin basic protein (MBP) for 5 min at 25 °C. (B) Time course of autophosphorylation and MBP phosphorylation of hMyo3A-KD mutants. Upper, phosphoimages; lower, input amount of hMyo3A-KD mutants and myelin basic protein (MBP) stained with Coomassie Brilliant Blue R-250. Reaction conditions are the same as in panel A.