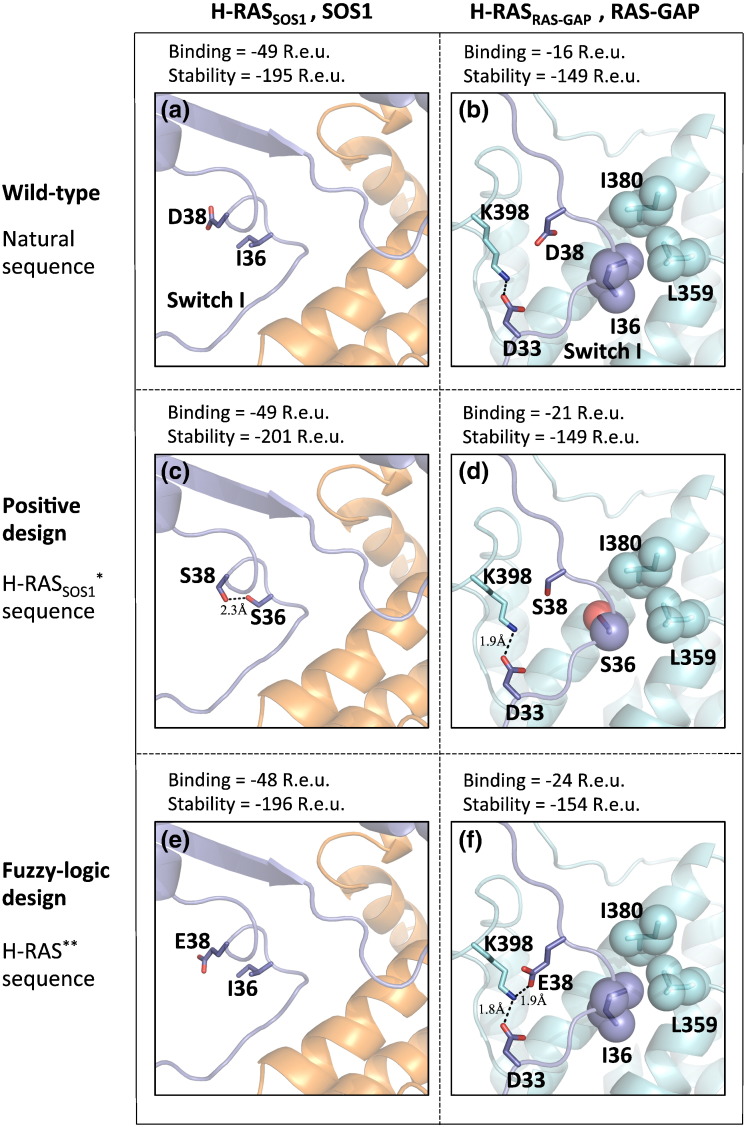
Fig. 3.
Single-state design versus fuzzy-logic design of the multispecific hub protein H-RAS. This view focuses on H-RAS switch I loop. The two H-RAS complexes presented here, H-RASSOS1 (a–e) and H-RASRAS-GAP (b–f), have different backbone conformations. Upper panel: structures of the native complexes of H-RASSOS1 (violet) and SOS1 (orange) complex (a) and H-RASRAS-GAP (violet) and RAS-GAP (cyan) complex (b), where I380, L359, and I36 form favorable van der Waals contacts and K398 and D33 form a salt bridge. Middle panel: single-state design of H-RASSOS1*,SOS1 complex, where D38S and I36S are introduced to form a hydrogen bond (c), and the designed sequence of H-RASSOS1* threaded on H-RASRAS-GAP backbone, where I36S packing with I380 and L359 is compromised (d). Bottom panel: fuzzy-logic design of H-RAS in complex with SOS1 (e) and with RAS-GAP (f). Position 36 was restored to Ile, which is the natural identity, while position 38 was designed to Glu, which forms a new electrostatic interaction with K398 in H-RASRAS-GAP*,RAS-GAP structure (f), resulting in a sequence that is similar to the wild type.