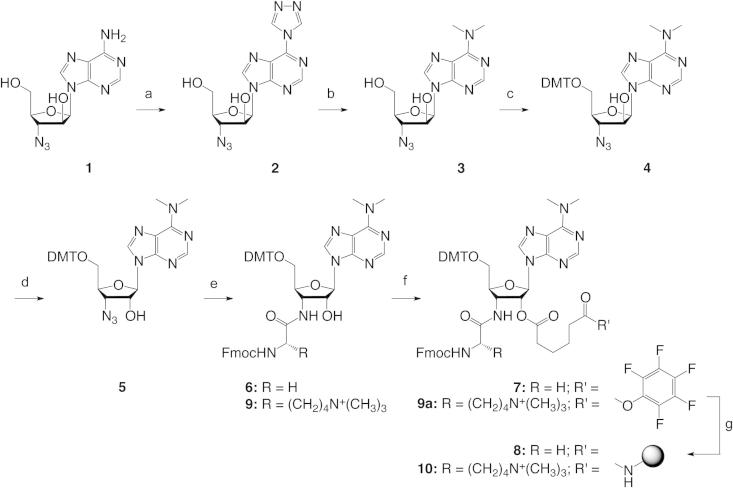
Scheme 1.
Synthesis of the modified solid supports 8 and 10. Reaction conditions: (a) 3.5 equiv N,N′-bis[(dimethylamino)methylene]-hydrazine (BDMAMH) dihydrochloride, 2.5 equiv Et3SiCl in pyridine, reflux, 24 h; (b) 40% aqueous dimethylamine, in pyridine, rt, 1 h, (72%, over (a) and (b)); (c) 1.2 equiv DMT-Cl, in pyridine, 16 h, rt, 93%; (d) i. 1.6 equiv trifluoromethanesulfonyl chloride, 1.6 equiv DMAP, 2.8 equiv (iPr)2NEt in CH2Cl2, 30 min, 0 °C, 84%, ii. 5.5 equiv CF3COO−K+, 2.0 equiv 18-crown-6, 2.5 equiv (iPr)2NEt in toluene, 16 h, 80 °C, 81%; (e) i. H2/Pd, ethyl acetate, 22 h, rt, 92%, ii. 1.3 equiv Fmoc-Gly-OPfp in DMF, rt, 5 h, 95% or 1.5 equiv Fmoc-Lys(CH3)3-OBt in DMF, rt, 12 h, 27%; (f) 3 equiv or 2 equiv of adipic acid bis(pentafluorophenyl)ester for 6 and 9 respectively, 1 equiv DMAP in N,N-dimethylformamide/pyridine (1/1, v/v), rt, 1 h, 51% for 7, (9a was used without isolation in the next step); (g) ∼1 equiv amino-functionalized polystyrene support (GE Healthcare, Custom Primer Support™ 200 Amino), pyridine, N,N-dimethylformamide, rt, 1 day, loading: 45 μmol/g for 8 and 18 μmol/g for 10. DMAP = 4-(N,N-dimethylamino)pyridine, Fmoc = N-(9-fluorenyl)methoxycarbonyl, Pfp = pentafluorophenyl.