Abstract
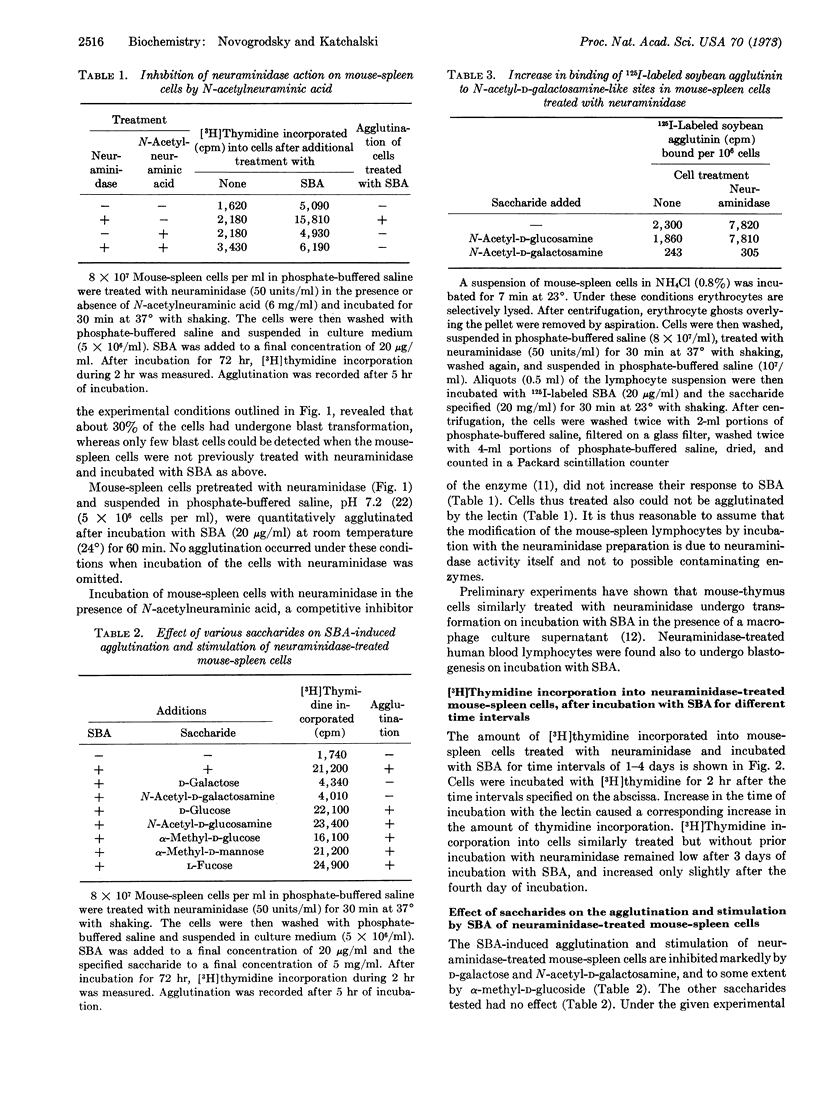
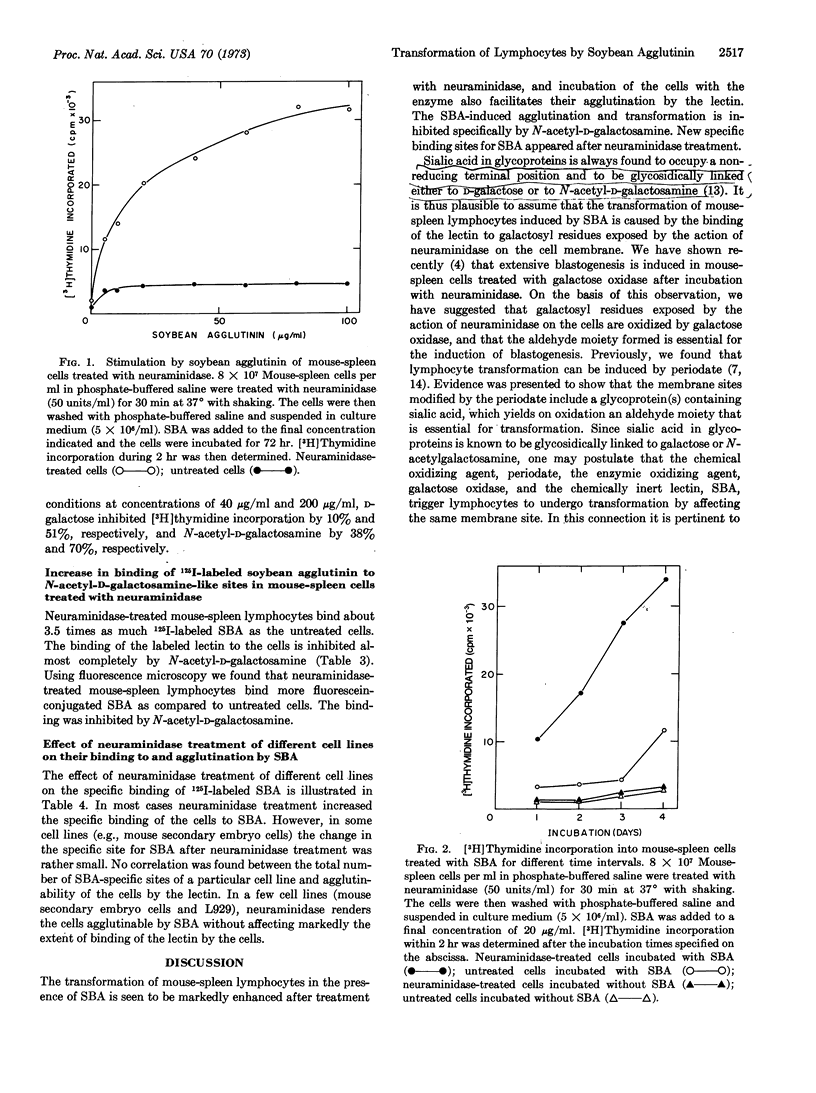
Transformation of mouse-spleen lymphocytes in the presence of soybean agglutinin is markedly enhanced after their treatment with neuraminidase (EC 3.2.1.18). Incubation of the cells with the enzyme also facilitates their agglutination by the lectin. The soybean agglutinin-induced agglutination and transformation is inhibited specifically by N-acetyl-D-galactosamine. New specific binding sites for soybean agglutinin were shown to appear after neuraminidase treatment. It is postulated that the transformation of neuraminidase-treated mouse-spleen lymphocytes induced by soybean agglutinin is caused by the binding of the lectin to galactosyl residues exposed by the action of neuraminidase on the cell membrane.
Keywords: mouse spleen, agglutination, membrane receptors, cell lines
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blume A., Gilbert F., Wilson S., Farber J., Rosenberg R., Nirenberg M. Regulation of acetylcholinesterase in neuroblastoma cells. Proc Natl Acad Sci U S A. 1970 Oct;67(2):786–792. doi: 10.1073/pnas.67.2.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger M. M. A difference in the architecture of the surface membrane of normal and virally transformed cells. Proc Natl Acad Sci U S A. 1969 Mar;62(3):994–1001. doi: 10.1073/pnas.62.3.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J. A., Sharon N., Lis H. Binding of soybean agglutinin by normal and trypsin-treated red blood cells. Biochim Biophys Acta. 1972 Apr 21;264(2):387–391. doi: 10.1016/0304-4165(72)90304-2. [DOI] [PubMed] [Google Scholar]
- Gordon Julius A., Blumberg Shmaryahu, Lis Halina, Sharon Nathan. Purification of soybean agglutinin by affinity chromatography On sepharose-N-epsilon-aminocaproyl-beta-D-galactopyranosylamine. FEBS Lett. 1972 Aug 1;24(2):193–196. doi: 10.1016/0014-5793(72)80765-8. [DOI] [PubMed] [Google Scholar]
- Greaves M., Janossy G. Elicitation of selective T and B lymphocyte responses by cell surface binding ligands. Transplant Rev. 1972;11:87–130. doi: 10.1111/j.1600-065x.1972.tb00047.x. [DOI] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. The structure of a phytohemagglutinin receptor site from human erythrocytes. J Biol Chem. 1970 May 25;245(10):2536–2545. [PubMed] [Google Scholar]
- Lis H., Sela B. A., Sachs L., Sharon N. Specific inhibition by N-acetyl-D-galactosamine of the interaction between soybean agglutinin and animal cell surfaces. Biochim Biophys Acta. 1970 Sep 15;211(3):582–585. doi: 10.1016/0005-2736(70)90265-8. [DOI] [PubMed] [Google Scholar]
- Novogrodsky A., Gery I. Enhancement of mouse thymus cells response to periodate treatment by a soluble factor. J Immunol. 1972 Dec;109(6):1278–1281. [PubMed] [Google Scholar]
- Novogrodsky A., Katchalski E. Effect of phytohemagglutinin and prostaglandins on cyclic AMP synthesis in rat lymph node lymphocytes. Biochim Biophys Acta. 1970 Aug 14;215(2):291–296. doi: 10.1016/0304-4165(70)90027-9. [DOI] [PubMed] [Google Scholar]
- Novogrodsky A., Katchalski E. Induction of lymphocyte transformation by sequential treatment with neuraminidase and galactose oxidase. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1824–1827. doi: 10.1073/pnas.70.6.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novogrodsky A., Katchalski E. Membrane site modified on induction of the transformation of lymphocytes by periodate. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3207–3210. doi: 10.1073/pnas.69.11.3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novogrodsky A., Katchlaski E. Induction of lymphocyte transformation by periodate. FEBS Lett. 1971 Jan 30;12(5):297–300. doi: 10.1016/0014-5793(71)80203-x. [DOI] [PubMed] [Google Scholar]
- Ozanne B., Sambrook J. Binding of radioactively labelled concanavalin A and wheat germ agglutinin to normal and virus-transformed cells. Nat New Biol. 1971 Aug 4;232(31):156–160. doi: 10.1038/newbio232156a0. [DOI] [PubMed] [Google Scholar]
- Richler C., Yaffe D. The in vitro cultivation and differentiation capacities of myogenic cell lines. Dev Biol. 1970 Sep;23(1):1–22. doi: 10.1016/s0012-1606(70)80004-5. [DOI] [PubMed] [Google Scholar]
- Schnebli H. P., Dukor P. Plant agglutinins used to distinguish between different classes of mouse lymphocytes. Eur J Immunol. 1972 Dec;2(6):607–609. doi: 10.1002/eji.1830020626. [DOI] [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- Spiro R. G. Glycoproteins. Annu Rev Biochem. 1970;39:599–638. doi: 10.1146/annurev.bi.39.070170.003123. [DOI] [PubMed] [Google Scholar]
- Toyoshima S., Fukuda M., Osawa T. Chemical nature of the receptor site for various phytomitogens. Biochemistry. 1972 Oct 10;11(21):4000–4005. doi: 10.1021/bi00771a025. [DOI] [PubMed] [Google Scholar]


