Abstract
Purpose: Transforming growth factor β1 (TGFβ1) is very important in the synthesis and degradation of extracellular matrix (ECM) and also in the mediation of human orbital fibroblasts (OFs) proliferation. MicroRNA-29 (MiR-29) plays an important role in this process. In the present study, the effects of TGFβ1 on the expression of miR-29 and whether miR-29 is involved in pro-survival signaling pathways mediated by TGFβ1 were examined in human OFs. Methods: Detecting the influence of TGFβ1 on the expression of miR-29a/b/c by real-time PCR analysis. Using 3-(4, 5-Dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) to detecting the influence of miR-29 on the increased proliferation caused by TGF-β1 on the human orbital fibroblasts. Using soft agar assay to detecting the influence of miR-29 on the increased colony formation caused by TGF-β1 on the human orbital fibroblasts. Western blot was used to detect the specific mechanisin. Results: TGFβ1 treatment decreases the expression of miR-29 in OFs. In the cultured OFs, the value of optical density (OD) in the group treated with miR-29 is lower than that in the group treated without miR-29 (P < 0.05). In the cultured OFs, the ratio of colony formation in the group treated with miR-29 is lower than that in the group treated without miR-29 (P < 0.05). In OFs, miR-29 decreases the secretion of Wnt3a and activation of β-catenin whether the treatment of TGFβ1 was used or not. MiR-29 decreases expression of Collagen, type I, alpha 1 (COL1A1) through down-regulation of wnt/β-catenin pathway. Conclusions: In OFs TGFβ1 treatment decreases expression of miR-29 which can cause the inhibition of normal ability of TGFβ1. MiR-29 inhibits TGFβ1-induced proliferation of OFS cell and decreases colony formation of OFS cell after TGFβ1 treatment. MiR-29 Mediates TGFβ1-induced Extracellular matrix synthesis through activation of Wnt/β-catenin pathway in human OFs.
Keywords: Orbital fibrosis, TGFβ1, miR-29, wnt/β-catenin pathway
Introduction
Extracellular matrix (ECM) is the dynamic micro-environment of human orbital fibroblasts (OFs) and can activate the original fibrosis pathway and matrix metalloproteinases to enhance the activity of collagenases [1,2]. Fibroblasts and myofibroblasts play a very important role in this pathological process by secreting growth factors, excess collagens and other matrix proteins [3].
Transforming growth factor β1 (TGFβ1) can cause phenotypic transformation of fibroblasts to myofibroblasts and plays a very important role in fibrosis [4,5]. It has been improved that TGFβ1 can cause phenotypic transformation of OFs to myofibroblasts, appearing as increased expression of α-smooth muscle actin and increased synthesis of ECM [6,7].
Micro-ribonucleic acids (miRNAs) are the little RNAs having regulating action. By inhibiting translation and deactivating target messenger ribonucleic acids, they can regulate the expression of many genes [8] and participate in a wide variety of biological processes including embryogenesis and disease [9-11]. More and more studies have confirmed that miRNAs play a vital role in the expression of genes associated with the fibrosis of organs (for examples: heart, kidney and liver) [12-15]. Two recent studies have also confirmed that let-7, miR-21 and miR-29 participate in the orbital fibrosis by regulating the expression of ECM and mediating the activation of TGFβ1 pathway [13,16].
Despite these studies, the molecular mechanisms of phenotypic transformation action of miR-29 have not been elucidated. We supposed that miR-29 was the important virulence regulator in matrix reorganization. To testing this hypothesis, we studied the influence of TGFβ1 on the expression of miR-29 in OFs, analyzed whether dose miR-29 regulate the proliferation of OFs caused by TGFβ1 and studied whether dose miR-29 regulate some signal pathways to regulate the expression of Collagen, type I, alpha 1 (COL1A1) to influence the phenotypic transformation mediated by TGFβ1.
Materials and methods
Reagents and cell line
Cell line of human orbital fibroblasts (OFs) was purchased from ATCC (Manassas, VA, USA) company.
TGF-β1 was purchased from PeproTech company. PCR primer and Qiazol reagent were purchased from Qiagen China (Shanghai, China) company. MiR-29 mimics and miR-29 inhibitors were purchased from Auragene Bioscience (Changsha, China) company. MTT dye was purchased from Sigma (St. Louis, MO, USA) company. Antibodies of collagen I, Wnt3a, β-catenin, phospho-β-catenin and actin was purchased from Abzoom biolabs (Dallas, USA) company. DKK1 (inhibitor of wnt/β-catenin pathway) was purchased from Xinyu Biological Technology (Shanghai, China) company.
Routine culture of OFs cell line
The human OFs cell line was purchased from ATCC and cultured according to the protocol recommended by ATCC. In short, the cells were maintained in MEM supplemented with 20% FBS. The cells were seeded in a 100-mm culture dish and were grown in a humidified 5% CO2 incubator. Medium was changed every three days. When the cells were grown to 90% confluence, they were washed with 1×PBS containing no Ca2+ or /Mg2+, dissociated with the trypsin-EDTA solution and passaged according to the 1 to 3 ratio. After 3 to the 5 passage, the cells were used to do the experiments. For TGF-β1 stimulation, cells were grown to about 80% confluence and incubated in serum-free medium overnight prior to each experiment. For the treatment of cells with miR-29 mimics, cells were treated with it 30 min before subjecting them to TGF-β1 stimulation.
Total RNA extraction
Using Qiazol reagent (according to the protocol recommended) to extract the total RNA of OFs. Using ND-1000 NanoDrop spectrophotometer to calculate and measure total RNA through A260. The microcapillary electrophoresis apparatus were used to evaluate the conformability of RNA.
Quantitative polymerase chain reaction (PCR)
Using miScript-Reverse transcription Kit and miRNA-SYBR Green PCR kit (Qiagen, USA) to measure miR-29 quantitatively. The first step included total RNA polyadenylation and reverse transcription and the second step was real time fluorescence quantitative PCR. Qiagen, chosen and purchased from GeneGlobe research center, was used in cDNA synthesis and synthesis of the primers of real time fluorescence quantitative PCR. According to the criteria of supplier, 2 ng sample of each group was used for test at two times. RNU6 was used as RNA reference to correct miRNA level of cells.
MTT assay
Using MTT analysis to evaluate the impact of miR-29 and TGFβ1 (10 ng/mL) on the proliferation of cells. Cells (8×103/hole) were seeded in 96-well culture plate and cultured with RPMI-1640 plus 10% FBS. At 24, 48, 72 and 96 hours after incubation, culture medium were stripped off. The cells were treated with 20 μl sterile MTT dye (5 mg/ml, Sigma, USA) at 37°C for 4 hours, and then were thoroughly mixed for half an hour after adding 200 μl DMSO. Micro plate reader was used to measure the optical density at 570 nm wavelength. In each group, three samples were measured to get the mean value.
Soft agar colony formation
Unconverted OFs or OFs treated with miR-29 were treated with TGFβ1 (10 ng/mL). They were subgrouped and mixed in 0.5 mL 0.35% agar containing growth medium and then were placed at plate containing 0.5% agar (to avoid fixed dependent cell growth). As the layers were solidified, 1 mL ordinary culture medium was added. Medium was changed every two days and this condition was maintained for 14 days. When the aggregation diameter of cells was larger than 100 μm, the clone was formed. At 14th day and 28th day, pictures were taken and the numbers of clones were counted.
Cell preparation for western blot analyses
The enough culture dishes were prepared according to the experimental design. When the confluence reached at 80%, each dish was treated according to the experimental design. The fundamental principles of first group: OFs were cultured in DMEM plus 10% FBS, and then were transfected with 50 nM miR-29a/b/c precursor or Lipofectamine 2000 (negative control). Six hours later, medium was changed to DMEM containing 0.1% FBS with or without 2 ng/ml TGFβ1 and the culture was kept for 24 hours. Last, the expression of β-catenin, p-β-catenin, wnt3a and β-actin was analyzed with Western Blot (WB). The fundamental principles of second group: OFs were cultured in DMEM plus 10% FBS, and then were transfected with 50 nM miR-29a/b/c precursor or Lipofectamine 2000 (negative control). 6 hours later, medium was changed to DMEM containing 0.1% FBS with or without 10 ng/mL DKK1 and the culture was kept for 24 hours. Last, the expression of COL1A1 was analyzed with WB.
Western blot analyses
Cells were lysed in RIPA buffer [150 mM NaCl, 100 mM Tris (pH 8.0), 1% Triton X-100, 1% deoxycholic acid, 0.1% SDS, 5 mM EDTA, and 10 mM NaF] supplemented with1 mM sodium vanadate, 2 mM leupeptin, 2 mM aprotinin, 1 mM phenylmethylsulfonyl fluoride (PMSF), 1 mMDTT, 2 mM pepstatin, and 1:100 protease inhibitor cocktailset III on ice for 30 min. After centrifugation at 4°C at 14,000 rpm for 8 min, the supernatant was harvested as the total cellular protein extracts and stored at -20°C. The protein concentration was determined using Bio-Rad protein assay reagent (Richmond, Virginia, USA). Running samples were prepared by adding a sample reducing agent and SDS sample buffer, incubating at 70°C for 10 min. Aliquots of protein extract samples were separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membrane. Membranes were blocked with 5% nonfat dry milk in 1×TBS containing 0.05% Tween-20 for 45 min, followed by incubation with antibodies. Protein bands were detected by incubation with horseradish peroxidase-conjugated antibodies (Pierce Biotechnology, Inc., Rockford, Illinois, USA) and visualized with SuperSignal West Pico chemiluminescence substrate (Thermo Scientific, Rockford, Illinois, USA) and then the results of WB were got.
Statistical analyses
Statistical significance was defined as P < 0.05. Data were expressed as the mean ± S.E. from a minimum of three independent experiments. Statistical analyses were performed with a completely random design one-way ANOVA.
Results
TGFβ1 treatment decreased the expression of miR-29 in OFs
At first, we detected whether did the TGFβ1 treatment change the expression of family members of miR-29 in OFs. In Figure 1, it can be seen that all three miR-29 family members of miR-29 are down-regulated obviously and time-dependently in OFs after treatment with TGFβ1 (10 ng/mL) for 48 hours (Figure 1).
Figure 1.
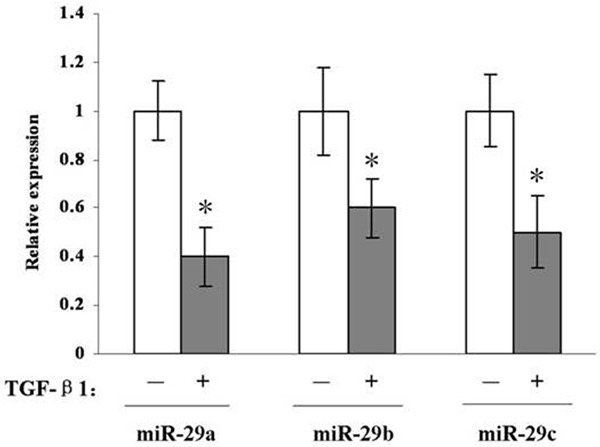
Real-time PCR analysis indicates that all three miR-29 family members are down-regulated in OFs after treatment with TGFβ1 for 48 h. *, P < 0.05 compared with untreated OFs.
MiR-29 depressed the proliferation of OFs caused by TGFβ1 stimulation
As we have seen [17], TGFβ1 plays an important role in the synthesis of ECM and proliferation of fibroblasts. In the first part, it has been proved that TGFβ1 treatment decreases the expression of miR-29 in OFs. MTT assay was used to detect that whether miR-29 affect the proliferation of OFs caused by TGFβ1 stimulation. OFs cells, cells treated with miR-29a/b/c mimics, cells treated with TGFβ1 and cells treated with both TGFβ1 and miR-29a/b/c mimics were seeded in 96-well plates and cells growth in 24 h, 48 h, 72 h or 96 h was monitored by MTT assay. In Figure 2, it can be seen that miR-29 down-regulates the OFs proliferation induced by TGFβ1. Therefore, miR-29 is the negative regulator of OFs proliferation, which indicates that miR-29 depresses the proliferation of OFs caused by TGFβ1 stimulation through blocking some signal pathways.
Figure 2.
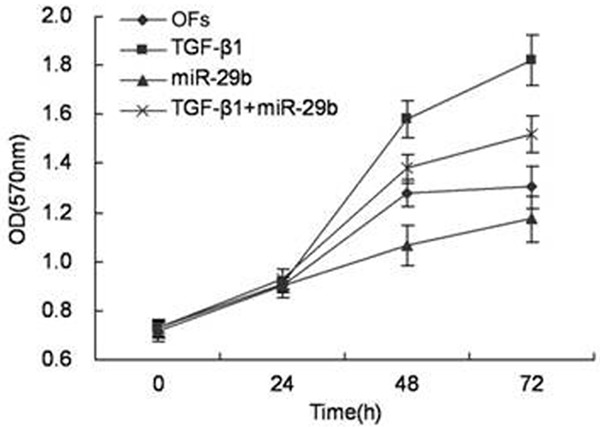
miR-29 down-regulated the process that TGFβ1 induced OFs proliferation. OFs cells treated miR-29a/b/c mimics, TGFβ1 or both of them, were seeded in 96-well plates and cell growth in 24 h, 48 h, 72 h or 96 h was monitored by MTT assay. (OFs; TGFβ1; miR-29a/b/c; miR-29a/b/c + TGFβ1).
MiR-29 decreased the cloning forming efficiency of OFs treated with TGFβ1
Clonig formation assay, based on the cloning formation ability of a single cell, is a method to determine the survival ability of cells in vitro. TGFβ1 or TGFβ1+miR-29 mimics were used to treat OFs and it was seen that overexpression of miR-29 depressed the proliferation of OFs obviously. In Figure 3, it can be seen that the cloning forming efficiency of the groups treated with TGFβ1+miR-29 mimics is about 30% of the efficiency of the control groups (treated with TGFβ1 solely). A possible explanation is that overexpression of miR-29 could inhibit the synthesis of ECM and proliferation of fibroblasts caused by TGFβ1.
Figure 3.
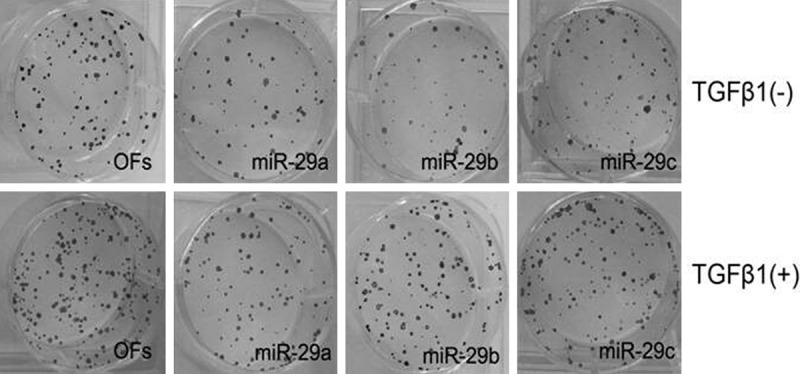
OFs colony formation induced by TGFβ1 is down-regulated by miR-29. OFs cells treated with miR-29a/b/c mimics, TGFβ1 or both of them were seeded in soft agar as described under Materials and Methods.
MiR-29 mediated the activation of wnt/β-catenin signaling pathway caused by TGFβ1
To study whether did miR-29 mediate the activation of wnt/β-catenin signaling pathway caused by TGFβ1, TGFβ1 was used to treat OFs group with high expression of miR-29 and the control group. Speaking concretely, OFs cells were cultured in DMEM supplemented with 10% FBS and were transfected with 50 nM miR-29a/b/c precursors, or a negative control, which had a scrambled sequence (control) using Lipofectamine 2000. After 6 h, the medium was changed to DMEM containing 0.1% FBS with or without 2 ng/ml TGFβ1, and the culture was continued for another 24 h. Western blot was used to analyze the expression of proteins of wnt/β-catenin signaling pathway after charge the cell solution. High expression of miR-29 suppresses the secretion of wnt3a and phosphorylation of β-catenin, whereas the total β-catenin protein level is coincident in each group (Figure 4).
Figure 4.
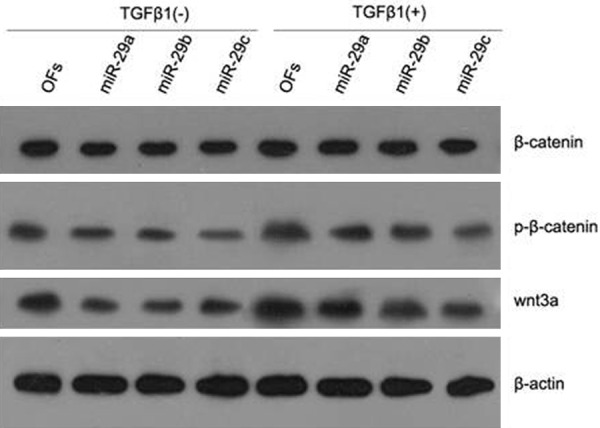
miR-29 mediates the process that wnt3a and phosphorylation of β-catenin induced by TGFβ1 in OFs. Effect of miR-29a/b/c precursors on the protein expression of β-catenin, p-β-catenin, wnt3a and β-actin in OFs in the presence (+) or absence (-) of TGFβ1 is shown. Expression of β-catenin and β-actin in all the groups is about the same. Expression of p-β-catenin and wnt3a is up-regulated by TGFβ1 and down-regulated by miR-29. Results are representative of those in three repeated experiments.
MiR-29 mediated the expression of COL1A1 through inhibiting the wnt/β-catenin signaling pathway
To study whether did miR-29 inhibit the expression of COL1A1 because of inhibiting the wnt/β-catenin signaling pathway, DKK1 (a kind of inhibitor of wnt/β-catenin signaling pathway) was used to treat OFs group or not. And then inhibitor of miR-29 was added in medium and Western blot was used to analyze the expression of COL1A1 in each group. The results of the tests substantiates that DKK1 inhibits the expression of wnt3a and inhibits the activity of β-catenin indirectly. As shown in Figure 5, the high expression of COL1A1 is detected in the group treated with miR-29 inhibitor and the expression of COL1A1 is depressed in the group treated with DKK1 compared with the control group. These results indicated that miR-29 decreased the expression of COL1A1 through inhibiting the wnt/β-catenin signaling pathway (Figure 5).
Figure 5.
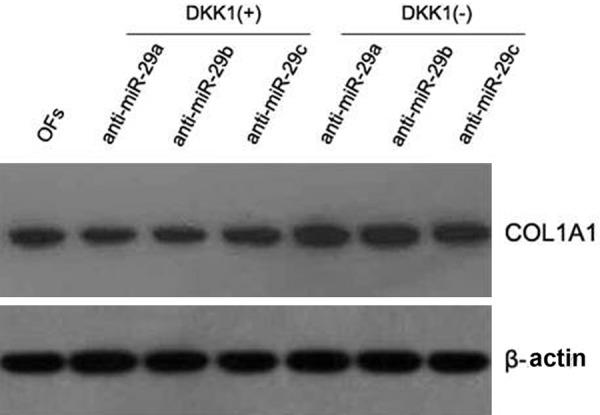
DKK1 inhibits anti-miR-29-induced expression of COL1A1 in OFs. OFs cells were cultured in DMEM supplemented with 10% FBS and were transfected with 50 nM anti-miR-29a/b/c precursors, or a negative control, which had a scrambled sequence (control) using Lipofectamine 2000. After 6 h, the medium was changed to DMEM containing 0.1% FBS with or without 10 ng/mL DKK1, and the culture was continued for another 24 h. Then, cells were lysed and analyzed by Western blot to determine expression levels of COL1A1. Results are representative of those in three repeated experiments.
Discussion
TGF-β is the important cytokine causing the fibrosis of organ or tissue. MiR-29 is the newly discovered micro RNA family, which is closely related with fibrotic diseases through inhibiting the expression of a variety of extracellular matrix proteins (including Collagen) and mediating many signaling pathways associated with fibrosis. There are reports indicate that TGFβ inhibits the expression of miR29-a/b/c. However, there are little reports associated with miR29 in human orbital fibroblasts (OFs). In this study, TGFβ1 was used to treat OFs in the experimental groups and Real Time Fluorescence Quantitative PCR was used to detect the difference of expression of miR-29 (including subfamily members of a/b/c). The results are objective and quantitative and are the objective basis to explore the function of miR-29.
It has been affirmed that TGFβ1, promoting the proliferation of OFs [18,19], is the key regulatory factor in the synthesis and degradation of extracellular matrix (ECM) .It also has been affirmed that miR-29 is important in the down regulation of some ECM (including Collagen [13]) and mediates many signaling pathways associated with fibrosis. Therefore, miR-29 is important in the functional integration of orbital fibrotic diseases [20] and might be the meaningful target of therapy.
Several studies have affirmed that the expression and activation of miR-29 are closely related with TGFβ1. For example, it has been confirmed that TGFβ1 can decreased the expression of miR-29 in some cells (including human liver fibroblasts, human epidermal keratinocy-tes, human cardiac fibroblasts and skeletal Muscle Cells) [12-16]. Because TGFβ1 is the central pathology regulator in orbital fibrosis [17,21], we assume that TGFβ1 may reduce the expression of miR-29, bring about the abnormal accumulation of ECM in human OFs and then play an important role in the orbital fibrosis. In this study, it has been confirmed that TGFβ1 can inhibit the expression of three family members of miR-29. Overexpression of miR-29 obviously inhibits the proliferation of human OFs caused by TGFβ1. The similar results are got after analyzing the same specimens using Soft Agar Colony Formation analysis and MTT analysis. Altogether, miR-29 participates in the proliferation of human OFs caused by TGFβ1 and suppresses the fibrosis through mediating the TGFβ1 signaling pathway.
To understand the mechanism of miR-29 regulating the proliferation of OFs caused by TGFβ1, the relative signaling pathway was researched. It has been found that TGFβ1 provokes the expression of COL1A1 and activates wnt3a/β-catenin signaling pathway. Inhibitor of Wnt lowers the expression of COL1A1 caused by TGFβ1. Similarly, transfection of miR-29 mimics lowers the expression of COL1A1 caused by TGFβ1. The results indicates that miR-29 decreases the expression of COL1A1 and inhibts the wnt3a/β-catenin signaling path-way. As a result, in human OFs, miR-29 mediates the synthesis of ECM caused by TGFβ1 via wnt3a/β-catenin signaling pathway. TGFβ1 can lowers the expression of miR-29, however, this does not mean that TGFβ1 regulates the expression directly. Although miR-29 regulates the synthesis of ECM via wnt3a/β-catenin signaling pathway, it does not mean that this pathway is exclusive because Smad, MAPK and PI3K-AKT signaling pathways may be associated with this phenomenon. Therefore, other signaling pathways should be investigated and altered expression of miR-29 may originate from aberration of many signaling pathways relevant to orbital fibrosis.
In conclusion, TGFβ1 increases the synthesis of ECM via activation of wnt3a/β-catenin signaling pathway, which is associated with the expression of miR-29 in human OFs. Our results indicate the new biological function of miR-29 and wnt3a/β-catenin signaling pathway: mediating the proliferation of human OFs and orbital fibrosis. These results also give the prompt that miR-29 and specific antagonists to relevant protein factors of wnt3a/β-catenin signal falls may be used to prevent and treat diseases of orbital fibrosis, which is a possible research directions in the future.
Disclosure of conflict of interest
None.
References
- 1.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 2.Thannickal VJ, Toews GB, White ES, Lynch JP 3rd, Martinez FJ. Mechanisms of pulmonary fibrosis. Annu Rev Med. 2004;55:395–417. doi: 10.1146/annurev.med.55.091902.103810. [DOI] [PubMed] [Google Scholar]
- 3.Bouzeghrane F, Reinhardt DP, Reudelhuber TL, Thibault G. Enhanced expression of fibrillin-1, a constituent of the myocardial extracellular matrix in fibrosis. Am J Physiol Heart Circ Physiol. 2005;289:H982–991. doi: 10.1152/ajpheart.00151.2005. [DOI] [PubMed] [Google Scholar]
- 4.Uhal BD, Kim JK, Li X, Molina-Molina M. Angiotensin-TGF-beta 1 crosstalk in human idiopathic pulmonary fibrosis: autocrine mechanisms in myofibroblasts and macrophages. Curr Pharm Des. 2007;13:1247–1256. doi: 10.2174/138161207780618885. [DOI] [PubMed] [Google Scholar]
- 5.Campbell SE, Katwa LC. Angiotensin II stimulated expression of transforming growth factor-beta1 in cardiac fibroblasts and myofibroblasts. J Mol Cell Cardiol. 1997;29:1947–1958. doi: 10.1006/jmcc.1997.0435. [DOI] [PubMed] [Google Scholar]
- 6.Lee CG, Cho S, Homer RJ, Elias JA. Genetic control of transforming growth factor-beta1-induced emphysema and fibrosis in the murine lung. Proc Am Thorac Soc. 2006;3:476–477. doi: 10.1513/pats.200603-040MS. [DOI] [PubMed] [Google Scholar]
- 7.Cutroneo KR, White SL, Phan SH, Ehrlich HP. Therapies for bleomycin induced lung fibrosis through regulation of TGF-beta1 induced collagen gene expression. J Cell Physiol. 2007;211:585–589. doi: 10.1002/jcp.20972. [DOI] [PubMed] [Google Scholar]
- 8.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taft RJ, Pang KC, Mercer TR, Dinger M, Mattick JS. Non-coding RNAs: regulators of disease. J Pathol. 2010;220:126–139. doi: 10.1002/path.2638. [DOI] [PubMed] [Google Scholar]
- 10.van Rooij E, Olson EN. MicroRNAs: powerful new regulators of heart disease and provocative therapeutic targets. J Clin Invest. 2007;117:2369–2376. doi: 10.1172/JCI33099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calin GA, Croce CM. MicroRNA-cancer connection: the beginning of a new tale. Cancer Res. 2006;66:7390–7394. doi: 10.1158/0008-5472.CAN-06-0800. [DOI] [PubMed] [Google Scholar]
- 12.Thum T, Gross C, Fiedler J, Fischer T, Kissler S, Bussen M, Galuppo P, Just S, Rottbauer W, Frantz S, Castoldi M, Soutschek J, Koteliansky V, Rosenwald A, Basson MA, Licht JD, Pena JT, Rouhanifard SH, Muckenthaler MU, Tuschl T, Martin GR, Bauersachs J, Engelhardt S. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature. 2008;456:980–984. doi: 10.1038/nature07511. [DOI] [PubMed] [Google Scholar]
- 13.van Rooij E, Sutherland LB, Thatcher JE, Di-Maio JM, Naseem RH, Marshall WS, Hill JA, Olson EN. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc Natl Acad Sci U S A. 2008;105:13027–13032. doi: 10.1073/pnas.0805038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chung AC, Huang XR, Meng X, Lan HY. miR-192 mediates TGF-beta/Smad3-driven renal fibrosis. J Am Soc Nephrol. 2010;21:1317–1325. doi: 10.1681/ASN.2010020134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chu AS, Friedman JR. A role for microRNA in cystic liver and kidney diseases. J Clin Invest. 2008;118:3585–3587. doi: 10.1172/JCI36870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pandit KV, Corcoran D, Yousef H, Yarlagadda M, Tzouvelekis A, Gibson KF, Konishi K, Yousem SA, Singh M, Handley D, Richards T, Selman M, Watkins SC, Pardo A, Ben-Yehudah A, Bouros D, Eickelberg O, Ray P, Benos PV, Kaminski N. Inhibition and role of let-7d in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2010;182:220–229. doi: 10.1164/rccm.200911-1698OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuentes-Calvo I, Blazquez-Medela AM, Eleno N, Santos E, Lopez-Novoa JM, Martinez-Salgado C. H-Ras isoform modulates extracellular matrix synthesis, proliferation, and migration in fibroblasts. Am J Physiol Cell Physiol. 2012;302:C686–697. doi: 10.1152/ajpcell.00103.2011. [DOI] [PubMed] [Google Scholar]
- 18.Chen SJ, Yuan W, Mori Y, Levenson A, Trojanowska M, Varga J. Stimulation of type I collagen transcription in human skin fibroblasts by TGF-beta: involvement of Smad 3. J Invest Dermatol. 1999;112:49–57. doi: 10.1046/j.1523-1747.1999.00477.x. [DOI] [PubMed] [Google Scholar]
- 19.Saika S, Yamanaka O, Baba Y, Kawashima Y, Shirai K, Miyamoto T, Okada Y, Ohnishi Y, Ooshima A. Accumulation of latent transforming growth factor-beta binding protein-1 and TGF beta 1 in extracellular matrix of filtering bleb and of cultured human subconjunctival fibroblasts. Graefes Arch Clin Exp Ophthalmol. 2001;239:234–241. doi: 10.1007/s004170100275. [DOI] [PubMed] [Google Scholar]
- 20.Cushing L, Kuang PP, Qian J, Shao F, Wu J, Little F, Thannickal VJ, Cardoso WV, Lu J. miR-29 is a major regulator of genes associated with pulmonary fibrosis. Am J Respir Cell Mol Biol. 2011;45:287–294. doi: 10.1165/rcmb.2010-0323OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moore BB, Hogaboam CM. Murine models of pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2008;294:L152–160. doi: 10.1152/ajplung.00313.2007. [DOI] [PubMed] [Google Scholar]


