Abstract
Transmembrane protease serine 4 (TMPRSS4) is a type-II transmembrane serine protease that plays an important role in the migration of cancer cells. This study aimed to investigate both the expression of TMPRSS4 and its clinical significance in prostate cancer. The expression of TMPRSS4 was evaluated in 73 pairs of prostate cancer and adjacent non-cancerous tissues by immunohistochemistry. The level of TMPRSS4 in prostate cancer tissues was significantly higher than that in adjacent non-cancerous tissues. High TMPRSS4 expression was significantly associated with advanced TNM stage and LNM. No association between TMPRSS4 expression and progression-free survival was observed in all patients. Stratified analyses according to clinical features revealed that patients with low TMPRSS4 expression had poor prognosis compared with those with high TMPRSS4 expression in subjects not receiving neoadjuvant chemotherapy. In conclusion, TMPRSS4 showed abnormal expression in prostate cancer tissues. TMPRSS4 may be a potential prognostic biomarker for prostate cancer patients who did not undergo neoadjuvant chemotherapy.
Keywords: Transmembrane protease, serine 4, prostatic cancer, prognosis, biomarker, neoadjuvant chemotherapy
Introduction
Prostate cancer is the most common malignant carcinoma in the male reproductive system. The incidence of prostate cancer increases with ageing. It has obvious regional difference, and is more prevalent across Europe and America [1]. Prostate cancer recently shows an increasing incidence in China, but it remains relatively low [2]. Prostate cancer is both a highly heterogeneous and aggressive disease [3]. Many newly diagnosed prostate cancers are indolent, but some patients may die of metastatic prostate cancer [4]. No effective treatment method for advanced prostate cancer exists, despite much advancement in prostate cancer treatment, over the past decades. The mechanisms that are involved in the progression and recurrence of prostate cancer remain unclear. Therefore, new prognostic biomarkers for identifying high-risk subpopulations of prostate cancer patients must be urgently developed to facilitate the design of patient-specific therapeutic regimen.
Type II transmembrane serine proteases (TTSPs) are family of proteolytic enzymes that are directly anchored to the plasma membranes [5]. It has been demonstrated that TTSPs participate in the cell signaling transduction and are implicated diverse range of physiological functions [6]. TTSPs can degrade extracellular matrix which increases both the migration and the diffusion abilities of cells in the tissues. Furthermore, the levels of TTSPs are increased during the growth and progression of tumor [7]. TTSPs may participate in proliferation, migration, and infection of tumor cells [6,8]. Transmembrane protease serine 4 (TMPRSS4), located on chromosome 11q23.3, is a member of the TTSPs family. TMPRSS4 encoding a polypeptide of 437 amino acid residues has trypsin activity. TMPRSS4 interacts with the molecules on cell membrane and in extracellular matrix, and participates in the growth, migration, and epithelial-mesenchymal transition (EMT) of tumor cells [9]. Therefore, it is closely related to tumor metastasis [10]. It has been found that TMPRSS4 is abnormally expressed in multiple tumor tissues [11-15]. In the present study, we evaluated TMPRSS4 expression in a total of 73 pairs of prostate cancer and adjacent non-cancerous tissues, and its association with clinical features and outcome of prostate cancer patients.
Materials and methods
Patients
A total of 73 prostate cancer patients, who received surgery resection in Shanghai Cancer Center between January 2007 and October 2008, were recruited to participate in the present study. The samples were fixed with 4% formaldehyde, and were treated with routine paraffin embedding. Pathologic examination was performed for all tissue samples by two pathologists to confirm the presence of prostate cancer. Patients with history of cancer, other than prostate cancer, were excluded. The time of biochemical recurrence was defined as the interval between the start date of the treatment and the first of two successive values of serum prostate-specific antigen (PSA) level ≥ 0.2 ng/ml. Each patient signed an informed written consent form. The use of human tissues for experimental procedures was approved by the Ethics Committee of Shanghai Cancer Center.
Tissue microarray (TMA)
Prostate cancer tissue chips were prepared by Shanghai Outdo Biotech Company, and the specific method was described as previous [16]. Briefly, the paraffin block of each donor was made into slices and stained by hematoxylin & eosin (H&E). By the positioning of target tissue under microscope, representative prostate cancer and benign tissues were selected. Using microarrayer (Beecher Instruments), the tissue core with the diameter of 2 mm was obtained by drilling on the paraffin block of donor, and then was inserted into the paraffin block of the receptor with a total of 146 lattices. Serial sections with the thickness of 6 μm were made. Pathologic diagnosis was performed for each point of TMA.
Immunohistochemistry (IHC)
Immunohistochemical assay was conducted in accordance to the manual of ABC (avidin-biotin complex) kit. The samples were placed in 3% H2O2 at room temperature for 10 min, after dewaxing and hydrating them with TMA, to remove endogenous peroxidase. Antigen was retrieved using 0.01 mol/L citrate buffer through high-pressure thermal retrieval method. The samples were sealed for 30 min, after adding rabbit serum. TMPRSS4 antibody was then added, with the titer of 1:4000. The sections were incubated in humid box overnight at 4°C. A ready-to-use secondary antibody was added to the sections, then, they were kept for 30 min at room temperature. DAB coloration and haematoxylin restaining were performed. After color separation, the section was gradient-dehydrated with alcohol, and transparentized with xylol. The section was sealed with Arabic gum. The samples were only considered valid if they were sufficient and easily observable. If the section was folded or unstained, or cancer cells could not be observed, the sample was considered as invalid. The invalid samples were therefore excluded from further analysis.
The IHC results were determined by two independent pathologists who were blinded to the clinicopatholigical data. The staining intensity was divided into four grades: 0, none; 1, mildly staining; 2, moderately staining; and 3, strong staining. The percentage scoring of cells showing positive TMPRSS4 was as follows: 0, no positive cell; 1, < 10%; 2, 11%-50%; 3, 51%-80%; 4, > 80%. The TMPRSS4 expression level was high if the product of the staining intensity and percentage scoring of cells was > 8, whereas the expression level was low if the product was ≤ 8.
Statistical analyses
All statistical analyses were performed using SPSS Version 19.0 Software (SPSS, Inc., Chicago, IL, USA). Mann Whitney U test was used to analyze the quantitative variables. Pearson’s χ2 test and Spearman’s correlation analysis were both used to determine the relationship between the TMPRSS4 expression and the clinicopathological parameters. Survival curves were plotted using the Kaplan-Meier method, and the differences between the groups were compared using the log-rank test. The statistical significance was determined at P < 0.05.
Results
Patient characteristics
A total of 73 prostate cancer patients were included in this retrospective study with enough tissue samples. The mean age was 73.7 years (range 59 to 82 years). Twenty nine patients (39.7%) had a serum SPA level that was greater than 20 ng/ml. Pathological T (pT) stage was pT2 in 62 cases (83.6%) and pT2 in 11 cases (15.1%). The Gleason Scores (GS) were < 7 in 28 cases (37.0%), 7 in 21 cases (28.8%), and > 7 in 19 cases (26.0%). Thirty three patients (45.2%) were treated with neoadjuvant chemotherapy. The clinicopathological characteristics of the patients are summarized in Table 1.
Table 1.
Clinicopathological characteristics of prostate cancer patients
| Characteristics | No. of patients | % |
|---|---|---|
| Age (years) | ||
| Mean | 73.7 ± 5.8 | |
| range | 59-82 | |
| PSA | 29.4 ± 31.1 | |
| GS | ||
| < 7 | 28 | 37.0 |
| 7 | 21 | 28.8 |
| > 7 | 19 | 26.0 |
| unknown | 6 | 8.2 |
| TNM stage | ||
| II | 61 | 83.6 |
| III | 10 | 13.7 |
| IV | 2 | 2.7 |
| pT stage | ||
| 2 | 62 | 84.9 |
| 3 | 11 | 15.1 |
| LNM | ||
| no | 71 | 97.3 |
| yes | 2 | 2.7 |
| Neoadjuvant chemotherapy | ||
| yes | 33 | 45.2 |
| no | 40 | 54.8 |
| Biochemical recurrence | ||
| absence | 33 | 45.2 |
| presence | 32 | 43.8 |
| unknown | 8 | 11 |
Association of TMPRSS4 expression with clinical features of prostate cancer patients
IHC results showed that TMPRSS4 was expressed in both prostate cancer and adjacent non-cancerous tissues, but with varying degrees (Figure 1). The levels of TMPRSS4 in prostate cancer tissues were obviously higher than those in adjacent non-cancerous tissues (P < 0.05). The patients were divided into two groups: the high and the low expression groups. High TMPRSS4 expression was significantly related to TNM stage (P = 0.042) and lymph node metastasis (LNM) (P = 0.012, Table 2). No significant association between the TMPRSS4 expression and other clinical features of prostate cancer patients was found.
Figure 1.
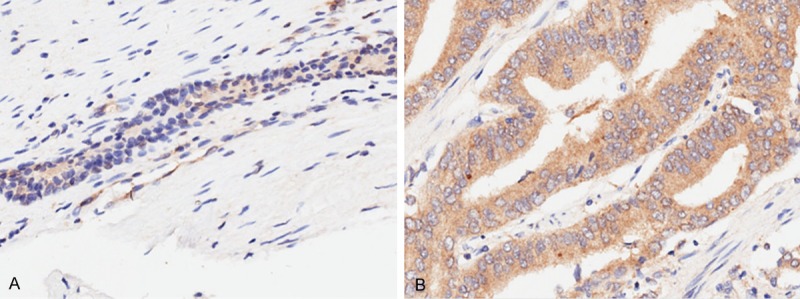
TMPRSS4 overexpression in archived paraffin-embedded prostate cancer tissues. Representative IHC images of TMPRSS4 expression in normal prostate (A) and prostate cancer tissues (B).
Table 2.
Association between TMPRSS4 expression and clinical features of prostate cancer patients
| Features | TMPRSS4 expression | P value | |
|---|---|---|---|
|
| |||
| High | Low | ||
| Age (years) | |||
| ≤ 70 | 3 | 16 | 0.293 |
| > 70 | 14 | 36 | |
| Gleason score | |||
| < 7 | 6 | 19 | 0.628 |
| 7 | 6 | 14 | |
| > 7 | 3 | 15 | |
| TNM stage | |||
| II | 13 | 44 | 0.042 |
| III | 2 | 8 | |
| IV | 2 | 0 | |
| pT categories | |||
| 2 | 14 | 44 | 0.825 |
| 3 | 3 | 8 | |
| LNM | |||
| no | 15 | 52 | 0.012 |
| yes | 2 | 0 | |
Prognostic significance of TMPRSS4 expression in prostate cancer patients
Progression-free survival (PFS) was used in the present study as a primary endpoint to evaluate the effect of TMPRSS4 on the prognosis in prostate cancer patients. The median PFS was 55 months (range 2 to 73 months). A total of 32 patients (43.8%) developed biochemical recurrence during the follow-up. Patients with high TMPRSS4 expression had a longer PFS than those with low TMPRSS4 expression (median PFS, 66 vs. 33 months), but the difference was not statistically significant (P = 0.120). We further evaluated the effect of TMPRSS4 expression on PFS by stratifying clinical features. Patients with high TMPRSS4 expression had a significant longer PFS than those with low TMPRSS4 expression in subjects without neoadjuvant chemotherapy (median PFS, 67 vs. 32 months; P = 0.041; Figure 2).
Figure 2.
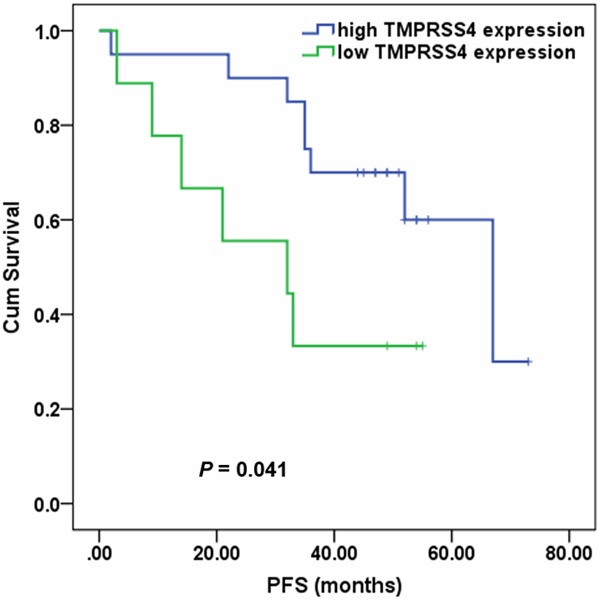
Kaplan-Meier analysis of PFS in prostate cancer patients without neoadjuvant chemotherapy according to TMPRSS4 expression.
Discussion
Previous studies have shown that TMPRSS4 is involved in EMT in cancer cells, and participated in cancer cell invasion, metastasis, and migration [10]. Furthermore, TMPRSS4 was described in a cell type-dependent manner to modulate cancer cell proliferation [10]. TMPRSS4 downregulated E-cadherin expression by inducing the transcriptional repressor SIP1/ZEB2 and promoted the occurrence of EMT in colon cancer cell lines [10]. Moreover, TMPRSS4 can induce integrin alpha5 expression and its signal transduction to downregulate E-cadherin, resulting in cancer invasion and concomitant EMT [17,18]. TMPRSS4 can activate some downstream signaling pathways, such as focal adhesion kinase (FAK), extracellular signal-regulated kinase (ERK), Akt, Src, and Rac1. The activation of FAK and ERK signaling pathway is necessary for the invasion and EMT induced by TMPRSS4 [18]. Min et al. [19] found that the induction and transcription of urokinase-type plasminogen activator (uPA) are necessary for the invasion induced by TMPRSS4 and the relevant signals. TMPRSS4 can induce the transcription of uPA by activating transcription factors, such as Sp1, Sp3, and AP-1 [20]. Moreover, it can activate the pro-uPA through its proteolytic activity [19]. In lung cancer and prostate cancer, the uPA expression shows significant correlation with TMPRSS4 expression [18]. Therefore, TMPRSS4 may mediate the invasion and metastasis of prostate cancer through uPA. However, the underlying molecular mechanism of TMPRSS4 in prostate cancer needs to be further clarified.
TMPRSS4 is overexpressed in many types of cancer, such as breast [11], pancreatic [12], gallbladder [15], colorectal [14], and gastric cancers [21]. Wu et al. [15] found in their study on gallbladder cancer that high TMPRSS4 expression of showed obvious correlation with tumor size, histological grade, TNM stage and LNM. Prognosis was poor for gallbladder cancer patients with high TMPRSS4 expression. Thus, TMPRSS4 may play an important role in the progression and metastasis of gallbladder cancer. Liang et al. [22] reported that breast cancer patients with low TMPRSS4 expression had longer survival time. In the present study, we found that TMPRSS4 expression was correlated with TNM and LNM. Although there was no association between TMPRSS4 expression and survival in all patients, high TMPRSS4 expression was significantly associated with longer PFS in patients not receiving neoadjuvant chemotherapy, which was inconsistent with previous studies [15,22]. TMPRSS4 expression is correlated with the degree of malignance of some cancers, such as colorectal, gastric, breast, and gallbladder cancers [14,15,22,23]. However, Riker et al. [24] found that the level of TMPRSS4 in early-stage melanoma was obviously higher than that in metastatic melanoma. These results indicate that the effect of TMPRSS4 varies with the type of tumor cells. The molecular mechanism of TMPRSS4 in tumors may be very complex. Thus, larger and well-designed further studies are needed to validate the present findings because of our small sample size.
In conclusion, our findings indicate that TMPRSS4 may serve as a prognostic biomarker for prostate cancer patients who do not undergo neoadjuvant chemotherapy. However, the role of TMPRSS4 in prostate cancer remains unclear. Thus, further research is needed to reveal how TMPRSS4 affects the invasion and metastasis of prostate cancer.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant No. 81202003).
Disclosure of conflict of interest
None.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Lu Z, Qi L, Bo XJ, Liu GD, Wang JM, Li G. Expression of CD26 and CXCR4 in prostate carcinoma and its relationship with clinical parameters. J Res Med Sci. 2013;18:647–652. [PMC free article] [PubMed] [Google Scholar]
- 3.MacVicar GR, Hussain MH. Emerging therapies in metastatic castration-sensitive and castration-resistant prostate cancer. Curr Opin Oncol. 2013;25:252–260. doi: 10.1097/CCO.0b013e32835ff161. [DOI] [PubMed] [Google Scholar]
- 4.Ilic D, Neuberger MM, Djulbegovic M, Dahm P. Screening for prostate cancer. Cochrane Database Syst Rev. 2013;1:CD004720. doi: 10.1002/14651858.CD004720.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Szabo R, Bugge TH. Type II transmembrane serine proteases in development and disease. Int J Biochem Cell Biol. 2008;40:1297–1316. doi: 10.1016/j.biocel.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 6.Hooper JD, Clements JA, Quigley JP, Antalis TM. Type II transmembrane serine proteases. Insights into an emerging class of cell surface proteolytic enzymes. J Biol Chem. 2001;276:857–860. doi: 10.1074/jbc.R000020200. [DOI] [PubMed] [Google Scholar]
- 7.Benaud CM, Oberst M, Dickson RB, Lin CY. Deregulated activation of matriptase in breast cancer cells. Clin Exp Metastasis. 2002;19:639–649. doi: 10.1023/a:1020985632550. [DOI] [PubMed] [Google Scholar]
- 8.Netzel-Arnett S, Hooper JD, Szabo R, Madison EL, Quigley JP, Bugge TH, Antalis TM. Membrane anchored serine proteases: a rapidly expanding group of cell surface proteolytic enzymes with potential roles in cancer. Cancer Metastasis Rev. 2003;22:237–258. doi: 10.1023/a:1023003616848. [DOI] [PubMed] [Google Scholar]
- 9.Kim S, Lee JW. Membrane Proteins Involved in Epithelial-Mesenchymal Transition and Tumor Invasion: Studies on TMPRSS4 and TM4SF5. Genomics Inform. 2014;12:12–20. doi: 10.5808/GI.2014.12.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jung H, Lee KP, Park SJ, Park JH, Jang YS, Choi SY, Jung JG, Jo K, Park DY, Yoon JH, Park JH, Lim DS, Hong GR, Choi C, Park YK, Lee JW, Hong HJ, Kim S, Park YW. TMPRSS4 promotes invasion, migration and metastasis of human tumor cells by facilitating an epithelial-mesenchymal transition. Oncogene. 2008;27:2635–2647. doi: 10.1038/sj.onc.1210914. [DOI] [PubMed] [Google Scholar]
- 11.Cheng D, Kong H, Li Y. TMPRSS4 as a poor prognostic factor for triple-negative breast cancer. Int J Mol Sci. 2013;14:14659–14668. doi: 10.3390/ijms140714659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wallrapp C, Hahnel S, Muller-Pillasch F, Burghardt B, Iwamura T, Ruthenburger M, Lerch MM, Adler G, Gress TM. A novel transmembrane serine protease (TMPRSS3) overexpressed in pancreatic cancer. Cancer Res. 2000;60:2602–2606. [PubMed] [Google Scholar]
- 13.Kebebew E, Peng M, Reiff E, Duh QY, Clark OH, McMillan A. ECM1 and TMPRSS4 are diagnostic markers of malignant thyroid neoplasms and improve the accuracy of fine needle aspiration biopsy. Ann Surg. 2005;242:353–361. doi: 10.1097/01.sla.0000179623.87329.6b. discussion 361-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang A, Zhou H, Zhao H, Quan Y, Feng B, Zheng M. High expression level of TMPRSS4 predicts adverse outcomes of colorectal cancer patients. Med Oncol. 2013;30:712. doi: 10.1007/s12032-013-0712-7. [DOI] [PubMed] [Google Scholar]
- 15.Wu XY, Zhang L, Zhang KM, Zhang MH, Ruan TY, Liu CY, Xu JY. Clinical implication of TMPRSS4 expression in human gallbladder cancer. Tumour Biol. 2014;35:5481–5486. doi: 10.1007/s13277-014-1716-4. [DOI] [PubMed] [Google Scholar]
- 16.Zhang X, He C, He C, Chen B, Liu Y, Kong M, Wang C, Lin L, Dong Y, Sheng H. Nuclear PKM2 expression predicts poor prognosis in patients with esophageal squamous cell carcinoma. Pathol Res Pract. 2013;209:510–515. doi: 10.1016/j.prp.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen TH, Weber W, Havari E, Connors T, Bagley RG, McLaren R, Nambiar PR, Madden SL, Teicher BA, Roberts B, Kaplan J, Shankara S. Expression of TMPRSS4 in non-small cell lung cancer and its modulation by hypoxia. Int J Oncol. 2012;41:829–838. doi: 10.3892/ijo.2012.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim S, Kang HY, Nam EH, Choi MS, Zhao XF, Hong CS, Lee JW, Lee JH, Park YK. TMPRSS4 induces invasion and epithelial-mesenchymal transition through upregulation of integrin alpha5 and its signaling pathways. Carcinogenesis. 2010;31:597–606. doi: 10.1093/carcin/bgq024. [DOI] [PubMed] [Google Scholar]
- 19.Min HJ, Lee MK, Lee JW, Kim S. TMPRSS4 induces cancer cell invasion through pro-uPA processing. Biochem Biophys Res Commun. 2014;446:1–7. doi: 10.1016/j.bbrc.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 20.Min HJ, Lee Y, Zhao XF, Park YK, Lee MK, Lee JW, Kim S. TMPRSS4 upregulates uPA gene expression through JNK signaling activation to induce cancer cell invasion. Cell Signal. 2014;26:398–408. doi: 10.1016/j.cellsig.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 21.Luo ZY, Wang YY, Zhao ZS, Li B, Chen JF. The expression of TMPRSS4 and Erk1 correlates with metastasis and poor prognosis in Chinese patients with gastric cancer. PLoS One. 2013;8:e70311. doi: 10.1371/journal.pone.0070311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liang B, Wu M, Bu Y, Zhao A, Xie F. Prognostic value of TMPRSS4 expression in patients with breast cancer. Med Oncol. 2013;30:497. doi: 10.1007/s12032-013-0497-8. [DOI] [PubMed] [Google Scholar]
- 23.Sheng H, Shen W, Zeng J, Xi L, Deng L. Prognostic significance of TMPRSS4 in gastric cancer. Neoplasma. 2014;61:213–217. doi: 10.4149/neo_2014_027. [DOI] [PubMed] [Google Scholar]
- 24.Riker AI, Enkemann SA, Fodstad O, Liu S, Ren S, Morris C, Xi Y, Howell P, Metge B, Samant RS, Shevde LA, Li W, Eschrich S, Daud A, Ju J, Matta J. The gene expression profiles of primary and metastatic melanoma yields a transition point of tumor progression and metastasis. BMC Med Genomics. 2008;1:13. doi: 10.1186/1755-8794-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]


