Abstract
Background: Multidrug resistance (MDR) is a serious problem in chemotherapy and is one of the main reasons for a poor outcome of gastric cancer. Study on the key proteins in multidrug resistance is necessary for the treatment of gastric cancer. Methods: The expression of ToPo II, MRP and GST-π in 119 gastric cancers was retrospectively examined, and the results were analyzed in correlation with clinicopathological data. ToPo II negative, MRP positive and GST-π positive were regarded as three risk factors which may be associated with chemotherapy resistance and poor prognosis. Patients were divided into two groups: high-risk group (≥2 risk factors) and the low-risk group (<2 risk factors), and the tumor recurrence and patients’ survival time of the two groups were also analyzed. Results: The positive rates of ToPo II, MRP and GST-π were 73.9%, 42.9% and 51.3%, respectively. The positively correlation between the expression of MRP and GST-π had been found. A significant correlation was shown between ToPo II expression and the level of differentiation. Significant differences with GST-π expression were also found in relation to the sex and differentiation. In the high-risk group, the 3-year survival rate of patients with/without chemotherapy were 62.1% and 52.0%, 5-year survival rates were 44.8% and 40.0%, but the difference was not statistically significant (P>0.05). In the low-risk group, the 3-year survival rate of patients with/without chemotherapy were 81.2% and 51.5%, 5-year survival rates were 71.9% and 45.5%, and the difference was statistically significant (P<0.05). Conclusions: Combined detection of MDR-related proteins ToPo II, MRP and GST-π may be prospectively valuable for postoperative individualized chemotherapy, and further predict the outcomes of gastric cancer patients.
Keywords: Stomach neoplasms, multidrug resistance-associated proteins, chemotherapy, prognosis
Introduction
Gastric cancer is one of the most common cancers worldwide, the incidence is particularly high in Asian countries, including China [1]. Patients are often at advanced stages when first diagnosed, and five-year survival rates after surgical resection have been reported to range from 20% to only 36% [2]. To improve its prognosis and prolong disease-free survival, chemotherapy involving drugs including fluorouracil, cisplatin, and either paclitaxel or epirubicin or others have been introduced postoperatively and preoperatively [3]. Unfortunately, the total survival of patients with gastric cancer undergoing surgery with chemotherapy has not been improved noticeably [4]. Some studies have shown that the occurrence of drug resistance, especially resistance to multiple drugs, decreases the chemotherapy efficacy in a large proportion of cases [5].
Multidrug resistance (MDR), either inherent or acquired, is a serious problem in chemotherapy and is one of the main reasons for a poor outcome [6]. There are quite a number of different mechanisms accounting for drug resistance, and MDR protein family plays an essential role [7]. Topoisomerase II (ToPo II), Multidrug resistance protein (MRP) and Glutathione S-transferase π (GST-π) were the basis of multidrug resistance in malignant tumors [8,9]. It had been confirmed that MRP and GST-π overexpression, and decreased expression of ToPo II was important mechanism mediated multidrug resistance [10]. Using immunohistochemical technique, this study was to determine the protein expressions of ToPo II, MRP and GST-π in gastric cancer tissues, and explored their expressions with clinicopathological factors and patients’ survival time, in order to offer individually tailored chemotherapeutic options and improve the prognosis.
Materials and methods
Patients
Between June 2004 to June 2006, a total of 119 patients who underwent radical gastrectomy at the department of abdominal surgery, Zhejiang cancer hospital were retrospectively analyzed. 77 cases were males and 42 cases were females, aged from 25 to 78 years (mean 57.3 ± 6.7 years). Lesions ≥5 cm were 76 cases and lesions <5 cm were 43 cases. Pathological diagnoses were poorly-differentiated adenocarcinoma in 37 cases (31.1%), moderately-differentiated adenocarcinoma in 31 cases (26.1%), well-differentiated adenocarcinoma in 51 cases (42.8%). Phase I/II were 39 cases and Phase III/IV were 80 cases, patients with/without lymph nodes metastases were 93 cases and 26 cases, respectively.
All patients did not receive preoperative chemotherapy or other treatment for the tumor, and some patients had adjuvant chemotherapy based on platinum and 5-Fluorouracil (5FU) for 4 to 6 cycles. Written informed consent was obtained from all the study participants. The study was approved by the Ethics Committee of Zhejiang Cancer Hospital.
Immunohistochemical staining
The antibodies used in this study were purchased from Beijing Biosynthesis Biotechnology Corporation (Beijing, China). Immunohistochemical staining was carried out on the formalin-fixed, 4 μm thick paraffin-embedded tissue specimens. Briefly, the paraffin embedded sections were dried overnight at 37°C and deparaffinized in xylol, rehydrated and incubated with methanol/0.3% H2O2 for 30 min to block endogenous peroxidase activity. Possible background staining was removed by applying normal goat serum, diluted 1:20 for 1 h. The sections were incubated with the primary antibody (50 μL, respectively) and kept at 4°C overnight in a humidified chamber. After rinsing in PBS, the biotinylated secondary antibody goat-anti-rat (50 μL, perspectively) were applied for 60 min at 37°C. Diaminobenzidine (DAB) was employed as a chromogen. The sections were then counterstained with hematoxylin. For the negative controls, the primary antibodies were replaced with PBS. Pancreas, colon samples were used as a positive control for Topo-II and MRP, and ovary samples for GST-π, respectively. The specimens were evaluated independently by two pathologists in a blind fashion. Only cells with brown-colored staining were considered as positive. The intensity of expression of MDR-related proteins were stratified into 4 categories scored as follows: 1) negative (-): no appreciable cytomembrane, nuclear or cytoplasmic staining or staining in <10% of neoplastic cells; 2) 1+: appreciable staining in 10-25% of neoplastic cells; 3) 2+: appreciable staining in 25-75% of neoplastic cells; 4) 3+: appreciable staining in >75% of neoplastic cells.
Patients follow-up
Patients received routine follow-up after radical gastrectomy. Once every quarter within two years and once every half year after two years (patients received chemotherapy were followed-up by chemotherapy cycles). All patients were tracked by direct evaluation or phone interview until death or until study end-date. ToPo II negative, MRP positive and GST-π positive were regarded as three risk factors which may be associated with chemotherapy resistance and poor prognosis. Patients were divided into two groups: the high-risk group (≥2 risk factors) and the low-risk group (<2 risk factors), and the tumor recurrence and patients’ survival time of the two groups were analyzed.
Statistical analysis
All the experiment data is integrated into a comprehensive data set. Numerical data were recorded directly and measurement data were described as median and range. Statistical analysis was performed on SPSS software version 16.0 (SPSS Inc. Chicago, IL), and P<0.05 was considered as statistically significant.
Results
Location and distribution of ToPo II, MRP and GST-π
The positive staining of ToPo II was recognized to be expressed in the cell nucleus (Figure 1A), while MRP and GST-π were expressed in the cytoplasm of malignant cells (Figure 1B, 1C). The characteristic distribution pattern of three proteins was scattered expression in tumor tissue, although small areas of diffused expression were also observed.
Figure 1.
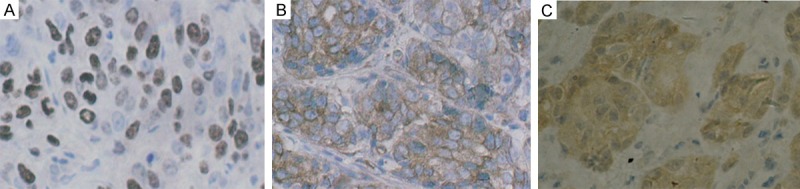
A. Immunohistochemical staining of ToPo II was identified in the cell nucleus (original magnification ×400). B. Immunohistochemical staining of MRP was recognized to be expressed in the cytoplasm of malignant cells (original magnification ×400). C. Immunohistochemical staining of GST-π was recognized to be expressed in the cytoplasm of malignant cells (original magnification ×400).
Expression of P-gp, LRP and MRP
In the 119 cases, the positive rate of ToPo II (73.9%) was significantly higher than MRP (42.9%) and GST-π (51.3%) (P<0.05). No significant difference between the expression of MRP (42.9%) and GST-π (51.3%) were observed (P>0.05), but we found the positive correlation between them (r=0.764) (Table 1).
Table 1.
Expression degree of MDR proteins
| MDR proteins* | Expression** | Positive numbers*** | |||
|---|---|---|---|---|---|
|
| |||||
| - | + | ++ | +++ | ||
| ToPo II | 31 (26.1%) | 61 (51.3%) | 18 (15.1%) | 9 (7.5%) | 88 (73.9%) |
| MRP | 68 (57.1%) | 46 (38.7%) | 4 (3.4%) | 1 (0.8%) | 51 (42.9%) |
| GST-π | 58 (48.7%) | 54 (45.4%) | 7 (5.9%) | 0 (0) | 61 (51.3%) |
r=0.764, the expression of MRP is correlated strong positively with GST-π;
P>0.05, MRP vs GST-π;
P<0.05, ToPo II vs MRP.
P<0.05, ToPo II vsGST-π; P>0.05, MRP vs GST-π.
Relationship between the clinicopathologic features and the expression of ToPo II, MRP and GST-π
Comparing the well, moderately and poorly differentiated degree, a significant correlation was shown between ToPo II expression and the level of differentiation (86.3%, 64.5% and 64.9%, respectively, P<0.05). Any significant differences with MRP expression were not found in relation to the clinicopathological factors. Significant differences with GST-π expression were found in relation to the sex (male vs. female, 59.7% vs. 35.7%, P<0.05) and differentiation (the well, moderately and poorly status, 64.7%, 41.9%, 40.5%, respectively, P<0.05) (Table 2).
Table 2.
Expression of ToPo II, MRP, GST-π and their relationship with clinicopathological factors
| Clinicopatho logic Features | Cases | ToPo II | MRP | GST-π | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| + (%) | χ2 | P | + (%) | χ2 | P | + (%) | χ2 | P | ||
| Sex | ||||||||||
| Male | 77 | 54 (70.1%) | 29 (37.7%) | 46 (59.7%) | ||||||
| Female | 42 | 34 (81.0%) | 1.65 | 0.198 | 2 (52.4%) | 2.40 | 0.121 | 15 (35.7%) | 6.27 | 0.012 |
| Age | ||||||||||
| ≤50 y | 33 | 24 (72.7%) | 16 (48.5%) | 14 (42.4%) | ||||||
| >50 y | 86 | 64 (74.4%) | 0.03 | 0.851 | 35 (40.7%) | 0.59 | 0.442 | 47 (54.7%) | 1.42 | 0.232 |
| Tumor size | ||||||||||
| ≥5 cm | 76 | 59 (77.6%) | 35 (46.1%) | 40 (52.6%) | ||||||
| <5 cm | 43 | 29 (67.4%) | 1.48 | 0.224 | 16 (37.2%) | 0.88 | 0.349 | 21 (48.8%) | 0.16 | 0.691 |
| Differentiation | ||||||||||
| Well | 51 | 44 (86.3%) | 20 (39.2%) | 33 (64.7%) | ||||||
| Moderately | 31 | 20 (64.5%) | 13 (41.9%) | 13 (41.9%) | ||||||
| Poorly | 37 | 24 (64.9%) | 7.04 | 0.029 | 18 (48.6%) | 0.79 | 0.672 | 15 (40.5%) | 6.47 | 0.039 |
| TNM staging | ||||||||||
| I, II | 39 | 25 (64.1%) | 17 (43.6%) | 16 (41.0%) | ||||||
| III, IV | 80 | 63 (78.8%) | 2.92 | 0.087 | 34 (42.5%) | 2.57 | 0.109 | 45 (56.3%) | 2.43 | 0.119 |
| Lymph node | ||||||||||
| Positive | 93 | 71 (76.3%) | 40 (43.0%) | 47 (50.5%) | ||||||
| Negative | 26 | 17 (65.4%) | 1.27 | 0.260 | 11 (42.3%) | 0.004 | 0.949 | 14 (53.8%) | 0.09 | 0.765 |
Relationship between the survival time and the expression of ToPo II, MRP and GST-π
The 3-and 5-year survival rates of the total 119 patients were 57.3% and 49.2%. No statistical difference was observed between single protein (ToPo II, MRP or GST-π) expression and the recurrence or survival time. Then patients were divided into two groups: the high-risk group (≥2 risk factors) and the low-risk group (<2 risk factors), the average recurrence time of the low-risk group was 21.29 ± 11.10 months, and was significantly longer than 15.16 ± 8.05 months of the high-risk group (P<0.01). The 3-year and 5-year survival rate of the high-risk group was 57.4% and 42.6%, however, it had no significant difference compared to 66.2% and 58.5% of the low-risk group (P>0.05).
In the high-risk group, the 3-year survival rate of patients with chemotherapy and patients without chemotherapy were 62.1% and 52.0%, and the 5-year survival rates were 44.8% and 40.0%, but the difference was not statistically significant (P>0.05) (Figure 2). In the low-risk group, the 3-year survival rate of patients with chemotherapy and patients without chemotherapy were 81.2% and 51.5%, the 5-year survival rates were 71.9% and 45.5%, and the difference was statistically significant (P<0.05) (Figure 3).
Figure 2.
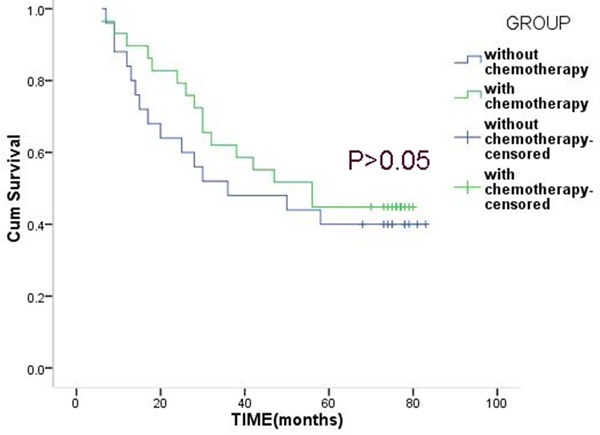
Overall survival of patients with or without chemotherapy in the high-risk group.
Figure 3.
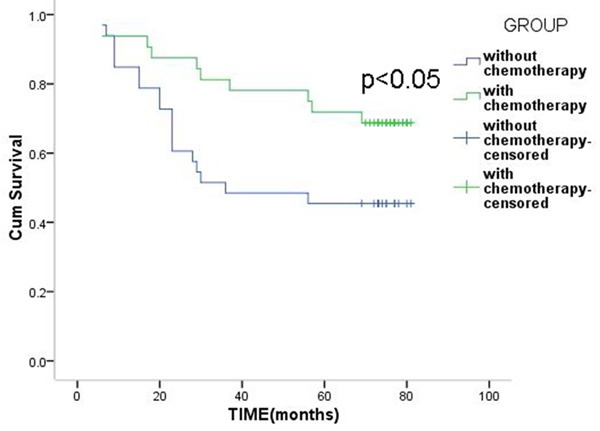
Overall survival of patients with or without chemotherapy in the low-risk group.
Discussion
Adjuvant chemotherapy after operation has been considered as a necessary mean to eliminate systemic micrometastases and remanent malignant cells to the fullest extent possible, ultimately improving survival [11,12]. Most patients, however, will experience relapse and treatment failure usually within 2~3 years after surgery. A major cause for such recurrence is the multidrug resistance (MDR) of malignant cells to different chemotherapeutic agents [13,14], which results from several molecular mechanisms. So, MDR is a challenge in cancer treatment, and detection of MDR genes or proteins may help guide adjuvant chemotherapy in gastric cancer and determine the prognosis of patients.
MRP, one of the most studied mechanisms about MDR, acts as an ATP-dependent outward transport pump and decreases intracellular accumulation of drugs by reducing co-transport mechanism of glutathione [15]. Several previous studies also indicated that overexpression of MRP most frequently predicts MDR. MRP confers resistance to alkylating agents, cyclophosphamide and other drugs [16]. GST-π is a multifunctional enzyme that plays a critical role in cellular detoxification by catalyzing the conjugation of reduced glutathione to hydrophobic and electrophilic compounds [17]. GST-π is considered to be associated with the efflux of cis-diaminodichloroplatin (CDDP), flurouracil and doxorubicin (DOX) through ATP-binding cassette transporters [18]. Topo-II is the target of several anticancer agents, such as doxorubicin, VM26, VP16 and mitoxantrone [19]. The decreased expression of ToPo II and changes of enzyme activity result in the dissociation of cleavable complex and reduced DNA damage, and finally cause the drug resistance [20].
Our study found these MDR proteins are interrelated, and MRP is correlated with GST-π (r=0.764). This finding suggests that synergistic effect of two or more proteins is the basis of MDR. Statistical analysis also indicates that none of the three proteins was significantly correlated with the recurrence and survival rates, so determination of single indicator is difficult to judge the effectiveness of adjuvant chemotherapy. Then, ToPo II negative, MRP positive and GST-π positive were regarded as three risk factors which may be associated with chemotherapy resistance and poor prognosis. Patients were divided into two groups: the high-risk group (≥2 risk factors) and the low-risk group (<2 risk factors). The recurrence time of the low-risk group was significantly longer than that of the high-risk group, suggesting that the decreased expression of ToPo II and high expression of MRP and GST-π was associated with tumor invasion, recurrence and poor prognosis, and this conclusion had been confirmed in the ovarian cancer [10]. In the low-risk group, the 3-year and 5-year survival rate of patients with chemotherapy was higher than that of the patients without chemotherapy. This results indicated that 5-Fu and platinum-based postoperative chemotherapy can increase survival benefits for patients in the low-risk group. Chemotherapy resistance did rarely exist in these patients, and in theory, postoperative chemotherapy could be done fully fit and the prognosis of the patient was significantly improved. In the high-risk group, the 3-year and 5-year survival rate of patients with chemotherapy was higher than that of the patients without chemotherapy, but the difference was not statistically significant. Therefore, the 5-Fu and platinum-based adjuvant chemotherapy did not improve the prognosis of the high-risk group, and for such patients, the postoperative chemotherapy needed to be carefully discussed and selected. These conclusions were based on a small number of cases and may have some limitations. Large sample of patients is being followed up in our center, and the detailed results will be reported in the near future.
For high-risk patients, due to the existence of MDR, benefit from chemotherapy was unsatisfactory. Inhibition of multidrug resistance proteins, or blocking drug resistance pathways may improve the chemotherapy efficacy, and finally improve the prognosis. Recent studies revealed some new methods to overcome MDR, such as tetrandrine and fangchinoline by inhibiting P-glycoprotein activity [21]. Shang showed that miR-508-5p could regulate multidrug resistance of gastric cancer by targeting ABCB1 and ZNRD1 [22]. Chen reported that pantoprazole (PPZ) pretreatment enhanced the cytotoxic effects of anti-tumor drugs on SGC7901 and reverse MDR of SGC7901/ADR by downregulating the V-ATPases/mTOR/HIF-1α/P-gp and MRP1 signaling pathway [23].
In conclusion, combined determination of MDR-related proteins ToPo II, MRP and GST-π may help tailor the chemotherapy regimes and predict the outcomes of treatment, although MDR remains a major challenge to effective chemotherapy. Further researches should focus on the combined detection of more sensitive proteins or molecular markers for individualized chemotherapy, and explore new methods to reverse or overcome MDR.
Acknowledgements
This study was supported by Natural Science Foundation of Zhejiang Province of China (No. LY14H160007).
Disclosure of conflict of interest
None.
References
- 1.Ferrari F, Reis MA. Study of risk factors for gastric cancer by population databases analysis. World J Gastroenterol. 2013;19:9383–91. doi: 10.3748/wjg.v19.i48.9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xia GG, Zhang CH, Wei ZW, Wu Y, He YL. Meta-analysis of adjuvant chemotherapy on prognosis for gastric cancer patients after D2 dissection. Zhonghua Wai Ke Za Zhi. 2013;51:447–51. [PubMed] [Google Scholar]
- 3.Geng M, Wang L, Li P. Correlation between chemosensitivity to anticancer drugs and Bcl-2 expression in gastric cancer. Int J Clin Exp Pathol. 2013;6:2554–9. [PMC free article] [PubMed] [Google Scholar]
- 4.Li X, Cao B, Liu Y, Mei L, Che X, Zhao Z. Multivariate analysis of prognostic factors in 549 patients undergoing surgical treatment of gastric cancer. Hepatogastroenterology. 2014;61:535–42. [PubMed] [Google Scholar]
- 5.Qiao W, Wang T, Zhang L, Tang Q, Wang D, Sun H. Association between single genetic polymorphisms of MDR1 gene and gastric cancer susceptibility in Chinese. Med Oncol. 2013;30:643. doi: 10.1007/s12032-013-0643-3. [DOI] [PubMed] [Google Scholar]
- 6.Xu HW, Xu L, Hao JH, Qin CY, Liu H. Expression of P-glycoprotein and multidrug resistance-associated protein is associated with multidrug resistance in gastric cancer. J Int Med Res. 2010;38:34–42. doi: 10.1177/147323001003800104. [DOI] [PubMed] [Google Scholar]
- 7.Hu WQ, Peng CW, Li Y. The expression and significance of P-glycoprotein, lung resistance protein and multidrug resistance-associated protein in gastric cancer. J Exp Clin Cancer Res. 2009;28:144. doi: 10.1186/1756-9966-28-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu D, Shi HC, Wang ZX, Gu XW, Zeng YJ. Multidrug resistance-associated biomarkers PGP, GST-pi, Topo-II and LRP as prognostic factors in primary ovarian carcinoma. Br J Biomed Sci. 2011;68:69–74. doi: 10.1080/09674845.2011.11730326. [DOI] [PubMed] [Google Scholar]
- 9.Kunjachan S, Rychlik B, Storm G, Kiessling F, Lammers T. Multidrug resistance: Physiological principles and nanomedical solutions. Adv Drug Deliv Rev. 2013;65:1852–65. doi: 10.1016/j.addr.2013.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao DY, Shen K, Yang JX, Guan J. The expression of MRP, GST-pi, Topo IIalpha and COX-2 in epithelial ovarian cancer and its relationship to drug resistance and prognosis. Zhonghua Yi Xue Za Zhi. 2007;87:1738–41. [PubMed] [Google Scholar]
- 11.Wu Y, Wei ZW, He YL, Schwarz RE, Smith DD, Xia GK, Zhang CH. Efficacy of adjuvant XELOX and FOLFOX6 chemotherapy after D2 dissection for gastric cancer. World J Gastroenterol. 2013;19:3309–15. doi: 10.3748/wjg.v19.i21.3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujitani K. Overview of adjuvant and neoadjuvant therapy for resectable gastric cancer in the East. Dig Surg. 2013;30:119–29. doi: 10.1159/000350877. [DOI] [PubMed] [Google Scholar]
- 13.Li Y, Tan BB, Zhao Q, Fan LQ, Liu Y, Hao YJ, Zhao XF. Tumor chemosensitivity is correlated with expression of multidrug resistance associated factors in variously differentiated gastric carcinoma tissues. Hepatogastroenterology. 2013;60:213–6. doi: 10.5754/hge12535. [DOI] [PubMed] [Google Scholar]
- 14.Zhu CY, Lv YP, Yan DF, Gao FL. Knockdown of MDR1 Increases the Sensitivity to Adriamycin in Drug Resistant Gastric Cancer Cells. Asian Pac J Cancer Prev. 2013;14:6757–60. doi: 10.7314/apjcp.2013.14.11.6757. [DOI] [PubMed] [Google Scholar]
- 15.Xu HW, Xu L, Hao JH, Qin CY, Liu H. Expression of P-glycoprotein and multidrug resistance-associated protein is associated with multidrug resistance in gastric cancer. J Int Med Res. 2010;38:34–42. doi: 10.1177/147323001003800104. [DOI] [PubMed] [Google Scholar]
- 16.Ge J, Chen Z, Wu S, Chen J, Li X, Li J, Yin J, Chen Z. Expression levels of insulin-like growth factor-1 and multidrug resistance-associated protein-1 indicate poor prognosis in patients with gastric cancer. Digestion. 2009;80:148–58. doi: 10.1159/000226089. [DOI] [PubMed] [Google Scholar]
- 17.Zhang KG, Qin CY, Wang HQ, Wang JX, Wang QM. The effect of TRAIL on the expression of multidrug resistant genes MDR1, LRP and GST-π in drug-resistant gastric cancer cell SGC7901/VCR. Hepatogastroenterology. 2012;59:2672–6. doi: 10.5754/hge11850. [DOI] [PubMed] [Google Scholar]
- 18.Gate L, Majumdar RS, Lunk A, Tew KD. Influence of glutathione S-transferase pi and p53 expression on tumor frequency and spectrum in mice. Int J Cancer. 2005;113:29–35. doi: 10.1002/ijc.20540. [DOI] [PubMed] [Google Scholar]
- 19.Chen W, Qiu J, Shen YM. Topoisomerase IIα, rather than IIβ, is a promising target in development of anti-cancer drugs. Drug Discov Ther. 2012;6:230–7. [PubMed] [Google Scholar]
- 20.Chau M, Christensen JL, Ajami AM, Capizzi RL. Amonafide, a topoisomerase II inhibitor, is unaffected by P-glycoprotein-mediated efflux. Leuk Res. 2008;32:465–73. doi: 10.1016/j.leukres.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 21.Sun YF, Wink M. Tetrandrine and fangchinoline, bisbenzylisoquinoline alkaloids from Stephania tetrandra can reverse multidrug resistance by inhibiting P-glycoprotein activity in multidrug resistant human cancer cells. Phytomedicine. 2014;21:1110–9. doi: 10.1016/j.phymed.2014.04.029. [DOI] [PubMed] [Google Scholar]
- 22.Shang Y, Zhang Z, Liu Z, Feng B, Ren G, Li K, Zhou L, Sun Y, Li M, Zhou J, An Y, Wu K, Nie Y, Fan D. miR-508-5p regulates multidrug resistance of gastric cancer by targeting ABCB1 and ZNRD1. Oncogene. 2014;33:3267–76. doi: 10.1038/onc.2013.297. [DOI] [PubMed] [Google Scholar]
- 23.Chen M, Huang SL, Zhang XQ, Zhang B, Zhu H, Yang VW, Zou XP. Reversal effects of pantoprazole on multidrug resistance in human gastric adenocarcinoma cells by down-regulating the V-ATPases/mTOR/HIF-1α/P-gp and MRP1 signaling pathway in vitro and in vivo. J Cell Biochem. 2012;113:2474–87. doi: 10.1002/jcb.24122. [DOI] [PMC free article] [PubMed] [Google Scholar]


