Abstract
Background: For the sake of reducing post extraction resorption, getting optimal positioning of the implant and shortening treatment time, immediate implant placement following tooth extraction has been proposed as a treatment option. However, the large bone defect peri-implant has a negative influence on the process of bone healing. In this study, umbilical cord mesenchymal stem cells (UCMSCs) were transplanted into the bone defect peri-implant inbeagle dogs and the effect of UCMSCs on bone regeneration in peri-implant were assessed. Methods: The mandibular second, third and fourth premolars of 8 beagle dogs were extracted bilaterally. The defects in one side were filled with platelet-rich fibrin (PRF) and then UCMSCs were injected into the defect area, while the defects in the other side were filled with PRF only as control group. The titanium implant was placed into the distal root socket of each extracted tooth. The animals were sacrificed at week 2, 4 and 8 post operative. The bone defects adjacent to the implant which are 4 mm in height, 4 mm in the mesio-distal direction and 3.5 mm in the bucco-lingual direction were made after immediate implant. Histomorphometric analysis was performed using methylene blue-fuchsin acid staining and hematoxylin and eosin (HE) staining to evaluate bone regeneration. Results: The direct bone-to-implant contact (BIC) in the experiment after 4 and 8 weeks was 56.47±1.18% and 76.23±2.08%; and in the control group was40.79±0.65% and 61.17±2.79%, respectively. The percentage of newly formed bone after 2, 4 and 8 weeks was 17.60±1.5%, 49.82±4.02% and 67.16±2.1% in experiment group; and in control group 14.30±1.25%, 37.04±2.29% and 58.83±3.36%, respectively. These results represented significant differences statistically. Conclusion: Intra-bone marrow injection of UCMSCs can promote new bone formation. UCMSCs can be used to as excellent seed cells to repair the large defect peri-implant after immediate implant.
Keywords: Umbilical cord mesenchymal stem cells, dental implant, peri-implant bone defect, immediate implant
Introduction
On account of implant denture possessing some outstanding advantages instance of artistic, high efficiency and no harm to adjacent teeth, it has been increasingly accepted by both doctors and patients. As a treatment option, immediate implant placement following tooth extraction has been recommended, particularly for the replacement of anterior teeth for several advantages such as optimal positioning of the implant, reduction of post extraction resorption and shortening the treatment time. However, the diameter of the socket being inconsistent with the implant usually generates a gap between the bony walls of the socket and the implant, where is the widest in the coronal part of the recipient site. Most studies indicated that marginal defects around implants (with all bony walls intact) less than 2 mm were thought to be negligible and had no need for bone augmentation [1-5].
In fact, most dentists often handle with large bony defect peri-implant which is more than 2 mm in clinical practice. In large bony defects, epithelial cells can be prior to colonizing the gap, which induces fibro integration but not osseointegration and results in implant failure at last [6,7]. In order to prevent fibro integration and achieve implant osseointegration at immediate implant, numerous investigations have been conducted. Guided bone regeneration (GBR) has become an important therapeutic procedure for bone and peri-implant defects, as well as bone augmentation procedures prior to implant placement [8]. GBR alone or in association with bone replacement graft had been recommended in gaps wider than 2 mm [9-12]. However, it has disadvantages like needing a second removal operation of non-resorbable membranes, being unable to produce sufficient bone formation with resorbable membranes such as collagen membrane.
The development of bone tissue engineering offers a hopeful opportunity for peri-implant bone defect repair. Three basic biological factors involved in bone tissue regeneration which are seed cells, differentiation inducing factor and scaffolding materials. Used as seed cells, mesenchymal stem cells come from bone marrow (BMSCs) has been used in the past years. But the drawbacks of its in collecting cells, aging, high viral pollution and limited proliferative property restrict the utility in bone tissue engineering [13].
As new and potential seed cells, UCMSCs have been paid extensive attention in tissue engineering. Besides its ease of isolation and no viral infection, UCMSCs are of higher proliferative capacity compared with BMSCs and have no drawbacks of embryonic stem cells such as source deficiency, xenoma rejection and ethical concerns. Previous studies demonstrated that UCMSCs could have potential for tissue engineering instance of cartilage repair and revascularization [14,15].
PRF, a second generation platelet concentrate, can be manufactured in a simple procedure. As containing varied growth factors such as platelet-derived growth factor (PDGF), fibrob last growth factors (FGFs), epidermal growth factor (EGF), transforming growth factor-beta (TGF-b) and so on. PRF has been widely used in bone tissue engineering. Animal experiments and clinical trials have proven that PRF has great potential in promoting the generation of the gingiva, alveolar bone and cementum in a variety of wound healing models [16,17]. Our previous work also showed PRF could promote the osseointegration of the immediate implants.
To our knowledge, there have been no studies investigating the effect of UCMSCs on repairing peri-implant bone defect post immediate implant. In this study, we created a bone defect adjacent to the implant after immediate implant. UCMSCs were injected into the defect. Then the peri-implant defects were filled with PRF in beagle dogs. The purpose of the present study was to evaluate the influence of UCMSCs on bone defect regeneration peri-implant after immediate implant.
Materials and methods
Animals and implants
Eight male beagle dogs of approximate 2 years old, weighing about 17-20 kg, were used in the experiment. The animals were fed a daily diet and kept in kennel cages in LUYE PHARMA (Yantai, Shandong province, China). They received appropriate veterinary care. All animals were in good general health, without any general occlusal trauma or oral viral or fungal lesions. SuperLine implants (Dentium Biomaterial Co., Ltd., Korea), 3.6 mm in diameter and 8 mm in length, were used in this experiment.
Preparation of PRF
After administration of local anesthesia, 10 ml of venous blood was drawn from the dogs’ forelimb. Then the blood without anticoagulant was centrifuged at 400 g for 10 min immediately in accordance with the manufacturer manual. PRF was obtained in the middle height of the tube.
Surgery and experimental procedures
After extracting the second, third and fourth premolars in bilateral mandible, dental implants were inserted into the distal root extraction socket immediately and the torque was 35 N.cm. Special care was taken to avoid injury to the bony wall and the dental implants. On the basis of implant surgical principles, bone defects peri-implant were created adjacent to the implant with 4 mm in height, 4 mm in the mesio-distal direction and 3.5 mm in the bucco-lingual direction after immediate implant (Figure 1). All dental implants gained the primal stability. In the experiment group, the 4th passage UCMSCs (Lifeline Cell Technology, FC-0020) re-suspended infresh α-MEM at a density of 1×108/ml, and 1×108 UCMSCs were injected into the marrow cavity of the defect area and filled with PRF. The peri-implant defects in the control group were filled with PRF only. The mucoperiosteal flaps were sutured with an interrupted absorbent suture. Antibiotic treatment by intramuscular injection of cephalosporin (15 mg/kg, b.i.d.) was continued for 72 hours after surgery. Animals were checked daily and fed with a soft diet. The animals were sacrificed 2 w, 4 w and 8 w after implantation by intraneous injection of overdosed sodium pentobarbital.
Figure 1.
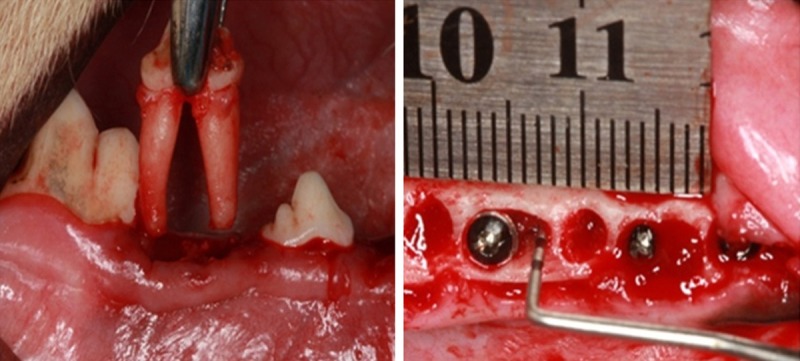
Extracted the third premolar, created defect in peri-implant.
Sample preparation
The mandibles were removed and dissected into 48 bony blocks containing the implants. There were 8 bony blocks in each group at every observing time point. All individual bony blocks containing the dental implants and the surrounding soft and hard tissue were fixed by 4% buffered formaldehyde solution. Five specimens were selected which were dehydrated in a graded series of ethanol and embedded in light-curing resin. The bony blocks were cut mid-axially in a buccal-lingual plane into 200 μm thick sections, using the cutting-grinding technique. The central section was harvested and then polished to a final thickness of approximately 40 μm (Exakt ®, Germany). Sections above were stained with methylene blue-fuchsin acid (Sigma). The other three bony blocks were demineralized in 10% EDTA for 4 months with constant stirring. After being washed in 0.1 M phosphate buffer (PH 7.2) the demineralized tissues were paraffin-embedded. Then the 5 μm-thick bone sections were mounted on glass slides to use for H&E-stained.
Histomorphometric analysis
All slides were observed and captured in the microscope (Olympus I×71, Japan). Histomorphometric measurement of the samples was conducted using Imaging software. BIC in each histological section was calculated by measuring the length of the implant surface in contact with bone tissue in comparison with the total length of the implant surface in the defect area, which was expressed by percentage. The percentage of newly formed bone was calculated by measuring the area of newly bone formation in comparison with the area of bone defect peri-implant. To calculate the new bone formation area, 3 sites were randomly selected for HE stained slide.
Statistical analysis
Mean values and standard deviations were calculated using a descriptive test for all of our experiment data. All data were subjected to paired t-test using SPSS 13.0 statistical software, and the significance level chosen was 5%.
Results
Macroscopic observations
All dogs tolerated the surgical procedure well, they were healthy during the entire experiment period. No conspicuous reduction in body weights and sign of postoperative infections were observed.
Histological findings
Two weeks after implanting, it showed that the defect area peri-implant filled with plenty of granulation tissue and inflammatory cells in the control group whereas in the experiment group few inflammatory, osteoblast and osteoclast were detected on the surface of the dental implant, little new bone could be observed in the bone defect area near the host bone. By the 4th week, numerous osteoblast and osteoclast were observed in both groups, and new bone could be detected not only near the host bone but also on the implant surface. Compared with the control group, the area of new bone was relative robust in the experiment. 8 weeks later, in the control group line of demarcation between the old and new bone was distinct. And in the experiment group, it is difficult to see the line of demarcation between the old and new bone in the defect area (Figure 2).
Figure 2.
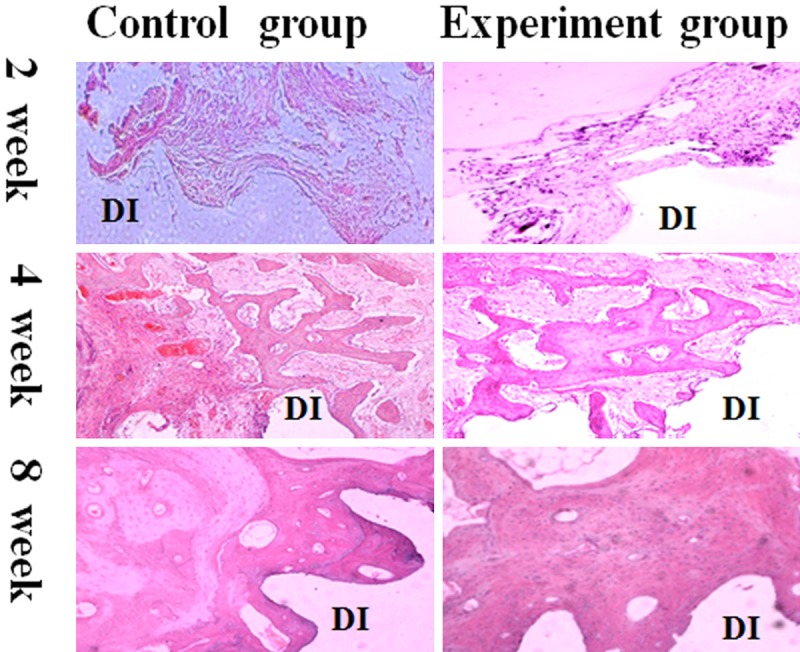
Histolgical observation and histomorphometric analysis in peri-implant defect by HE stained sections. Observation under the 100× magnification. After implanting 2 weeks, it is showed that the defect area peri-implant covered with abundant of granulation tissue and inflammatory cells the control group whereas in the experiment group few inflammatory, osteoblast and osteclast were detected near the dental implant, meanwhile, little new bone could be seen near the old bone. In the 4th week, numerous osteoblast and osteclast were observed in both groups, but the control group showed bony trabeculae was slender than the experiment group. In the 8th week, the line of demarcation between the old and new bone in the control group was distinct. And in the experiment group, it is difficult to see the line of demarcation between the old and new bone in the defect area peri-implant. DI: dental implant.
Histomorphometric analysis (Figure 4, Tables 1 and 2)
Figure 4.
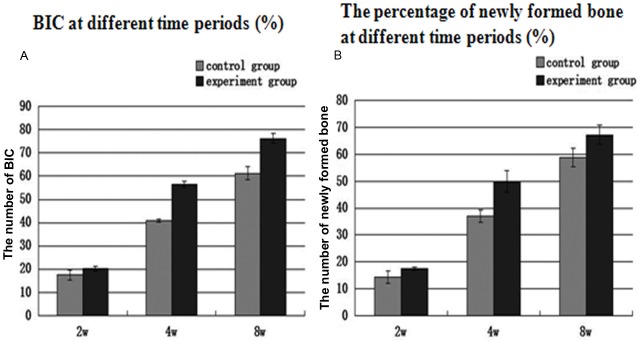
A: BIC measurement at different time. In the 4th and 8th week, the significances were different between the experiment group and control group; B: The percentage of newly formed bone at different time periods (%). At all time points, the significances were different between the experiment group and control group.
Table 1.
BIC measurements at different time periods (%) (n=5)
| 2 weeks | 4 weeks | 8 weeks | |
|---|---|---|---|
| Control group | 17.51±2.15 | 40.79±0.65★ | 61.17±2.79▲ |
| Experiment group | 20.33±0.87 | 56.47±1.18★ | 76.23±2.08▲ |
Significantly different from control group at 4 week (P < 0.05).
Significantly different from control group at 8 week (P < 0.05).
Table 2.
The percentage of newly formed bone at different time periods (%) (n=9)
| 2 week | 4 week | 8 week | |
|---|---|---|---|
| Control group | 14.30±1.25* | 37.04±2.29★ | 58.83±3.36▲ |
| Experiment group | 17.42±0.59* | 49.82±4.02★ | 67.16±3.60▲ |
Significantly different from control group at 2 week (P < 0.05).
Significantly different from control group at 4 week (P < 0.05).
Significantly different from control group at 8 week (P < 0.05).
Osteoblast proliferation was detected in the test group at the early stage of new bone formation. By the second week, almost no new bone tissue was visible on the surface of dental implant in the defect area in both groups. However, some newly bone formation could be detected at the bottom of the defect area and near the host bone. The BIC in the 2nd week showed experiment group (20.33±0.87%) was similar to control group (17.51±2.15%) and there was no significant difference between the two groups. There was a significant difference in newly formed bone area between the two groups, which was 17.60±1.5% in the experiment group and 14.30±1.25% in the control group.
In the 4th week, the quantity of newly formed bone in contact with dental implant surface was increased at this period in both groups. Most of the bone-implant interfaces contact directly, mass of newly formed bone occupied the defect region in experiment group. In control group, reticular formation by new formed trabecular structures occupied the defect area. BIC measurements in the experiment group were 56.47±1.18% and the control group was 40.79±0.65%, indicating a significant difference between both groups. The percentage of newly formed bone in the experiment group was 49.82±4.02% and the control group was 37.04±2.29%, indicating a significant difference as well. In the 8th week, bone tissue was more mature than before. In experiment group, bone to implant interface was closely contacted, and Haversian system could be identified. BIC measurements was 76.23±2.08% in the experiment group and 61.17±2.79% in the control group, showing a significant difference between groups. The percentage of newly formed bone was 67.16±2.1% in the experiment group and 58.83±3.36% in the control group, showing significant difference as well (Figures 2 and 3).
Figure 3.
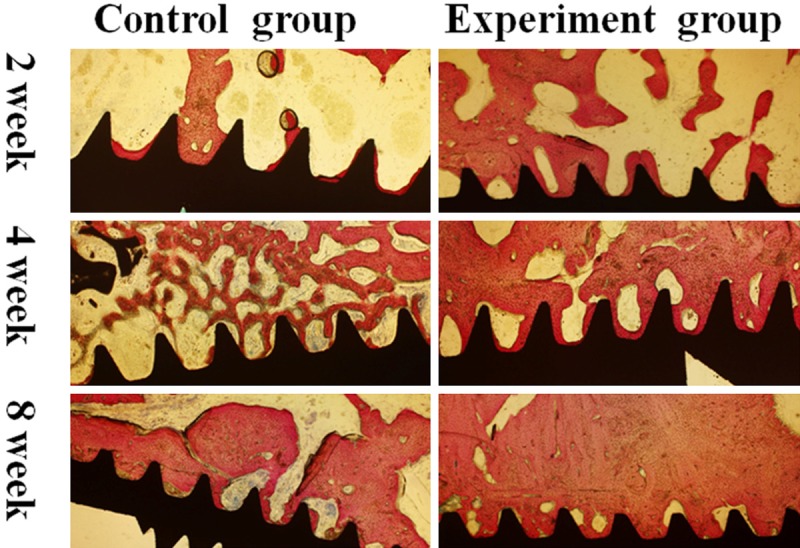
Histolgical observation and histomorphometric analysis in peri-implant defect by sections of bone tissue combined with dental implant. Original magnification, ×40. In the 2nd week, most of the bone-implant interface without close contact. In the 4th week, most of the bone-implant interface contact directly, mass of newly formed bone can be seen near the interface in experiment group. In control group, reticular formation by new formed trabecular structures within previous bone cavity. In the 8th week, bone tissue was more mature than previous. In experiment group, bone to implant interface was closely contacted, Haversin system could be identified.
Discussion
During the installation of dental implants, dentists usually have to deal with bone defects, especially in immediate implant. To accelerate the bone healing around the implant as well as get better implant osseointegration, multiple researches have been done in the last decade. For example, much attention was paid to changing the shape of implants and modifying the surface properties of the dental implants. The development of bone tissue engineering offers a new idea for peri-implant bone defect repair.
As seed cells, UCMSCs have irreplaceable advantages such as higher proliferative capacity, easy way of accessibility, and so on. Stem cells applied to bone defect repair were required to have the property of populating in the defect region easily and producing the best possible therapeutic effects quickly. Accumulating evidence in experiment and clinical treatment suggested that transplant stem cell intra-bone marrow is a powerful strategy for cellular therapy. As a consequence, we transplanted UCMSCs intra-bone marrow by injection, which made UCMSCs much more efficient to reach the bone defect area and improved research efficiency to the maximum extent.
Traditionally, hard tissue slices containing the implants and the surrounding soft and bone tissue were manufactured to observe the osseo-implant interface. Osseointegration or direct bone-to-implant contact is crucially important to succeed in establishing dental implants in the biological and clinical. In this experiment, BIC measurements had an increasing trend in both groups during the period of experimental time and there were significant differences between control group and experiment group in the 4th and 8th week (Figure 4, Tables 1 and 2). It is obvious that bone tissue was more mature in the experiment group (Figure 3). It is confirmed that UCMSCs had the talent of accelerating bone repair. Though there was no significant difference in BIC measurements between control group and experiment group in the 2nd week, the percentage of newly formed bone was significant difference and active bone reconstruction could be observed using HE staining (Figure 2). 2 weeks after implantation, some newly formed bone was observed in the experiment group and multiple osteoblasts were detected lining in chain. By contrast, the defect area in the control group was filled with granulation tissue and a great deal of inflammatory cells.
It has been reported that there are two kinds of bone formation, distal osteogenesis and contact osteogenesis, exist in bone-implant interface during the bone healing process, which form toward each other to promote osseointegration [18]. In the current study, 2 weeks post implanted, there was little new bone on the surface of host bone in the experiment group. However, a few newly formed bones were found on the surface of the implants in both groups. On the basis of this outcome, it could be assumed that the bone defect peri-implant interfered with the contact osteogenesis in the bone healing period of 2 weeks. It is worth mentioning that transplanted UCMSCs accelerate distal osteogenesis at the same time. Therefore, it is likely that UCMSCs can promote the bone healing by influencing the distal osteogenesis in the defect area peri-implant at the early stage of bone reconstruction.
It is well known that bone healing around the implant is a dynamic process accompanied with angiogenesis, osteogenesis and immune system activation, which is similar to bone injury repair. Tooth extract and dental implants embedding are acute injury to humans which can recruit mesenchymal stem cells derived from wound site as well as blood circulation to participate in the process of bone remodeling. Once we injected the UCMSCs into the defect area of peri-implant, and the transplanted stem cells are to act as an initiator to trigger the repair process of endogenous stem cell-based tissue. And more cells recruited, activated, and differentiated are involved in repairing bone defect, resulting in newly bone formation in the defect area peri-implant. It could be due to transplanted UCMSCs not only participate directly in bone rebuilding but recruit host bone progenitor cells through secretion of trophic factors to promote bone regeneration [19,20].
All in all, it is feasible and effective to apply bone tissue engineering to accelerate bone healing in large defect peri-implant in clinic. Seed cells, as one of the three elements of tissue engineering, are fundamental to bone reconstruction. Our finding showed that UCMSCs had the talent of accelerating bone formation in the large defect peri-implant. The mechanism of UCMSCs mediating bone tissue regeneration should be paid more attention in the future.
Acknowledgements
The authors would also like to acknowledge the grant support from the central laboratory of BinzhouMedicalUniversity.
Disclosure of conflict of interest
None.
References
- 1.Hammerle CHF, Jung RE, Feloutzis A. A systematic review of the survival of implants in bone sites augmented with barrier membranes (guided bone regeneration) in partially edentulous patients. J Clin Periodontol. 2002;29:226–31. doi: 10.1034/j.1600-051x.29.s3.14.x. [DOI] [PubMed] [Google Scholar]
- 2.Botticelli D, Berglundh T, Buser D, Lindhe J. The jumping distance revisited: an experimental study in the dog. Clin Oral Implants Res. 2003;14:35–42. doi: 10.1034/j.1600-0501.2003.140105.x. [DOI] [PubMed] [Google Scholar]
- 3.Covani U, Cornelini R, Barone A. Bucco-lingual bone remodeling around implants placed into immediate extraction sockets: a case series. J Periodontol. 2003;74:268–73. doi: 10.1902/jop.2003.74.2.268. [DOI] [PubMed] [Google Scholar]
- 4.Chen ST, Wilson TG, Hammerle CH. Immediate or early placement of implants following tooth extraction: review of biologic basis, clinical procedures, and outcomes. Int J Maxillofac Implants. 2004;19:12–25. [PubMed] [Google Scholar]
- 5.Jung UW, Kim CS, Choi SH, Cho KS, Inoue T, Kim CK. Healing of surgically created circumferential gap around non-submerged-type implants in dogs: a histomorphometric study. Clin Oral Implants Res. 2007;18:171–8. doi: 10.1111/j.1600-0501.2006.01310.x. [DOI] [PubMed] [Google Scholar]
- 6.Paolantonio M, Dolci M, Scarano A. Immediate implantation in fresh extraction sockets. A controlled clinical and histological study in man. J Periodontol. 2001;72:1560–71. doi: 10.1902/jop.2001.72.11.1560. [DOI] [PubMed] [Google Scholar]
- 7.Nemcovsky CE, Moses O, Artzi Z, Gelernter I. Clinical coverage of dishcence defects in immediate implant procedure: Three surgical modalities to achieve primary soft tissue closure. Int J Oral Maxillofac Implants. 2000;15:843–52. [PubMed] [Google Scholar]
- 8.Hammerle CH, Lang NP. Single stage surgery combining transmucosal implant placement with guided bone regeneration and bioresorbable materials. Clin Oral Implants Res. 2001;12:9–18. doi: 10.1034/j.1600-0501.2001.012001009.x. [DOI] [PubMed] [Google Scholar]
- 9.Cornelini R, Cangini F, Martuscelli G, Wennstrom J. Deproteinized bovine bone and biodegradable barrier membranes to support healing following immediate placement of transmucosal implants: a short-term controlled clinical trial. Int J Periodontics Restorative Dent. 2004;24:555–63. [PubMed] [Google Scholar]
- 10.Chen ST, Darby IB, Reynolds EC. A prospective clinical study of non-submerged immediate implants: clinical outcomes and esthetic results. Clin Oral Implants Res, 2007;18:552–62. doi: 10.1111/j.1600-0501.2007.01388.x. [DOI] [PubMed] [Google Scholar]
- 11.Polyzois I, Renvert S, Bosshardt DD, Lang NP, Claffey N. Effect of Bio-Oss® on osseointegration of dental implants surrounded by circumferential bone defects of different dimensions: an experimental study in the dog. Clinical Oral Implants Research. 2007;18:304–10. doi: 10.1111/j.1600-0501.2007.01207.x. [DOI] [PubMed] [Google Scholar]
- 12.Chen ST, Buser D. Clinical and esthetic outcomes of implants placed in postextraction sites. IntJ Oral Maxillofac Implants. 2009;24:186–217. [PubMed] [Google Scholar]
- 13.Baksh D, Yao R, Tuan RS. Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells. 2007;25:1384–92. doi: 10.1634/stemcells.2006-0709. [DOI] [PubMed] [Google Scholar]
- 14.Wen Y, Jiang B, Cui J, Li G, Yu M, Wang F, Zhang G, Nan X, Yue W, Xu X, Pei X. Superior osteogenic capacity of different mesenchymal stem cells for bone tissue engineering. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;116:e324–e32. doi: 10.1016/j.oooo.2012.02.024. [DOI] [PubMed] [Google Scholar]
- 15.Anusaksathien O, Giannobile WV. Growth factor delivery to re-engineer periodontal tissues. Curr Pharm Biotechnol. 2002;l3:129–39. doi: 10.2174/1389201023378391. [DOI] [PubMed] [Google Scholar]
- 16.Giannobile WV, Hernandez RA, Finkelman RD, Ryan S, Kiritsy CP, D'Andrea M, Lynch SE. Comparative effects of platelet-derived growth factor-BB and insulin-like growth factor-I, individually and in combination, on periodontal regeneration in Macaca fascicularis. J Periodontal Res. 1996;31:301–12. doi: 10.1111/j.1600-0765.1996.tb00497.x. [DOI] [PubMed] [Google Scholar]
- 17.Dohan DM, Choukroun J, Diss A. Platelet-rich fibrin (PRF): a second generation platelet concentrate. Part I: technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radio Endod. 2006;101:e37–e44. doi: 10.1016/j.tripleo.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 18.Puleo DA, Nanci A. Understanding and controlling the bone- implant interface. Biomaterials. 1999;20:2311–21. doi: 10.1016/s0142-9612(99)00160-x. [DOI] [PubMed] [Google Scholar]
- 19.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–84. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 20.Meirelles S, Fontes AM, Covas DT, Caplan AI. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev. 2009;20:419–27. doi: 10.1016/j.cytogfr.2009.10.002. [DOI] [PubMed] [Google Scholar]


