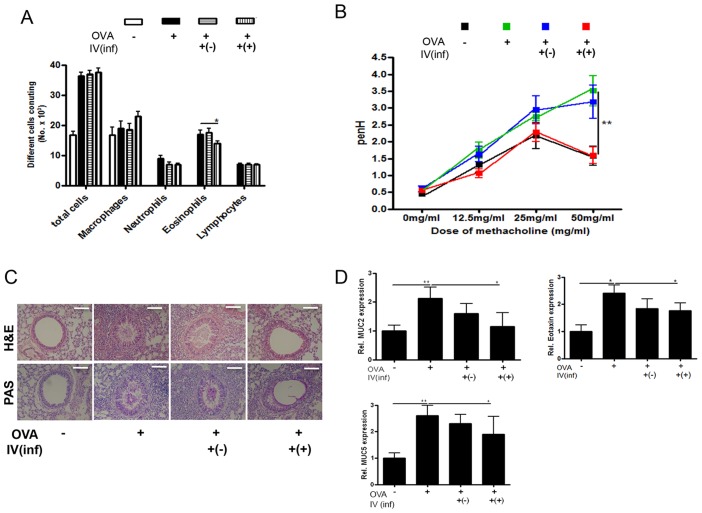
Figure 2. Amelioration of airway inflammation by CD4+Foxp3+T cell adoptive transfer before asthma induction (Stage I).
The number of inflammatory cells in the BALF samples was counted after Diff-Quik staining (A). The enhanced pause (PenH) was evaluated at baseline and after treatment with increasing doses of aerosolized methacholine (0–50 mg/mL). square; control mice, triangle; Ova-Alum treated mice, inverted triangle; OVA-Alum treated mice that receive Treg cells from uninfected mice, diamond; OVA-Alum treated mice that receive Treg cells from T.spiralis infected mice (B). The histological appearance of lungs after challenge with OVA and cell transfer (bar = 100 µm). The thin sections of lung were then stained with hematoxylin-eosin (H&E) and PAS stains (C). Relative quantification of MUC2 MUC5 and eotaxin gene expression in lung after the induction of airway inflammation (D). Total RNA was extracted from lung tissue and cDNA was synthesized. The gene expression levels of MUC2, MUC5 and eotaxin in the lungs of each group were analyzed using real-time PCR. The GAPDH gene was used as a control. Data are representative of three independent experiments [OVA-; PBS treated mice, OVA+; allergic airway inflammation-induced mice, IV(inf)+(-); CD4+Foxp3+T cell of normal mice adoptive transferred mice, IV(inf)+(+); CD4+Foxp3+T cell of T. spiralis-infected mice adoptive transferred mice, *p<0.05, **p<0.01, n = 6 mice/group].