Abstract
A simple heme-imidazole compound, having the same geometry as the heme-imidazole complex in myoglobin, has been synthesized. This compound, ferropyrroporphyrin-N-[3-(1-imidazolyl)propyl]amide, reversibly binds oxygen in the solid state or when dissolved in a polystyrene film. These results suggest that the principal factors governing reversible oxygen binding are the electronic nature of the base (imidazole), neighboring-group effects of the basic group, and immobilization of the heme group.
Keywords: hemoglobin, oxyheme, heme-oxygen bond, neighboring group
Full text
PDF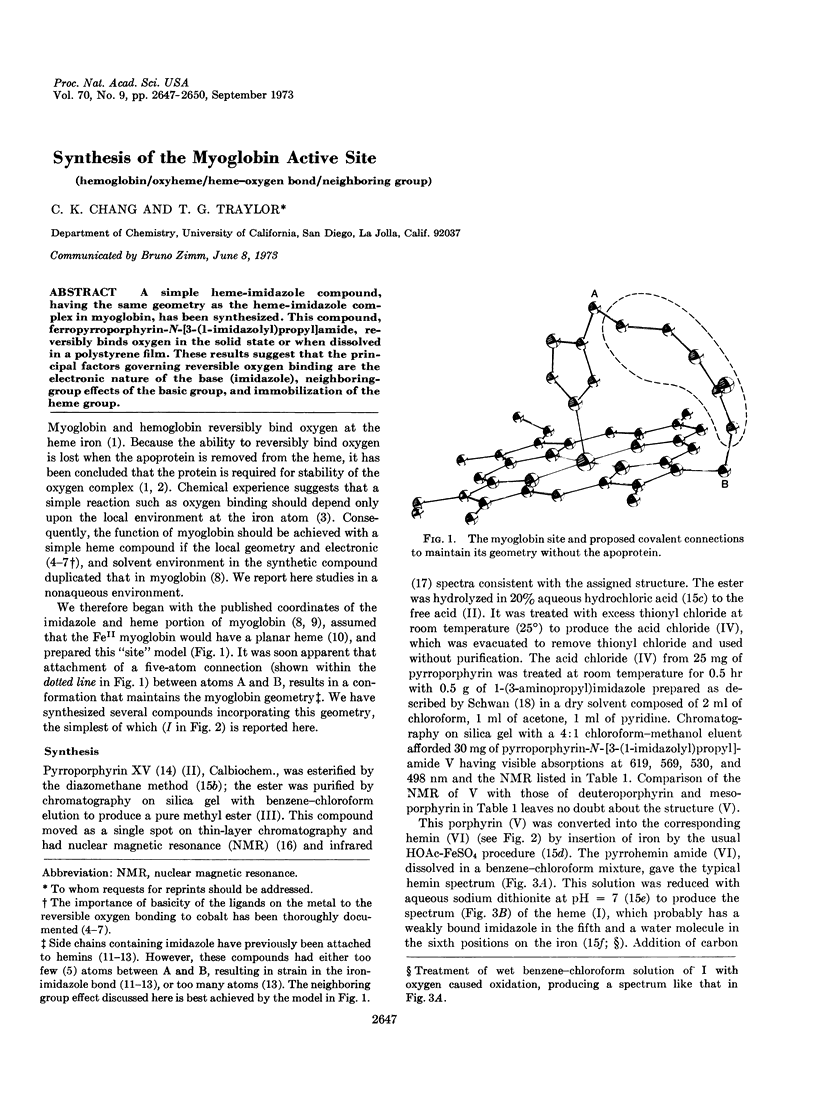

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRAUNITZER G., HILSE K., RUDLOFF V., HILSCHMANN N. THE HEMOGLOBINS. Adv Protein Chem. 1964;19:1–71. doi: 10.1016/s0065-3233(08)60188-6. [DOI] [PubMed] [Google Scholar]
- Caughey W. S., Alben J. O., Fujimoto W. Y., York J. L. Substituted deuteroporphyrins. I. Reactions at the periphery of the porphyrin ring. J Org Chem. 1966 Aug;31(8):2631–2640. doi: 10.1021/jo01346a042. [DOI] [PubMed] [Google Scholar]
- Dickmann H., Chang C. K., Traylor T. G. Cyclophane porphyrin. J Am Chem Soc. 1971 Aug 11;93(16):4068–4070. doi: 10.1021/ja00745a053. [DOI] [PubMed] [Google Scholar]
- LOSSE G., MUELLER G. [On organic catalysts. 67. The catalytic activation of hemin by histidine derivatives and histidine peptides]. Hoppe Seylers Z Physiol Chem. 1962 May 4;327:205–216. doi: 10.1515/bchm2.1962.327.1.205. [DOI] [PubMed] [Google Scholar]
- Perutz M. F. Stereochemistry of cooperative effects in haemoglobin. Nature. 1970 Nov 21;228(5273):726–739. doi: 10.1038/228726a0. [DOI] [PubMed] [Google Scholar]
- ROSSIFANELLI A., ANTONINI E., CAPUTO A. HEMOGLOBIN AND MYOGLOBIN. Adv Protein Chem. 1964;19:73–222. doi: 10.1016/s0065-3233(08)60189-8. [DOI] [PubMed] [Google Scholar]
- Sano S., Shingu T. The chemical structure of pemptoporphyrin. Biochem J. 1965 Oct;97(1):250–256. doi: 10.1042/bj0970250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stynes D. V., Stynes H. C., Ibers J. A., James B. R. Kinetics of the reaction of amine complexes of cobalt(II) protoporphyrin IX dimethyl ester with oxygen. Evidence for hydrogen bonding with coordinated oxygen. J Am Chem Soc. 1973 Feb 21;95(4):1142–1149. doi: 10.1021/ja00785a024. [DOI] [PubMed] [Google Scholar]
- Tauzher G., Amiconi G., Antonini E., Brunori M., Costa G. Thermodynamics of the reversible oxygenation of N,N-ethylene bis (salicylideneiminato) cobalt(II) in pyridine. Nat New Biol. 1973 Feb 14;241(111):222–223. doi: 10.1038/newbio241222a0. [DOI] [PubMed] [Google Scholar]
- Walker F. A. Reactions of monomeric cobalt-oxygen complexes. I. Thermodynamics of reaction of molecular oxygen with five- and six-coordinate amine complexes of a cobalt porphyrin. J Am Chem Soc. 1973 Feb 21;95(4):1154–1159. doi: 10.1021/ja00785a026. [DOI] [PubMed] [Google Scholar]
- Warme P. K., Hager L. P. Heme sulfuric anhydrides. II. Properties of heme models prepared from mesoheme sulfuric anhydrides. Biochemistry. 1970 Mar 31;9(7):1606–1614. doi: 10.1021/bi00809a020. [DOI] [PubMed] [Google Scholar]


