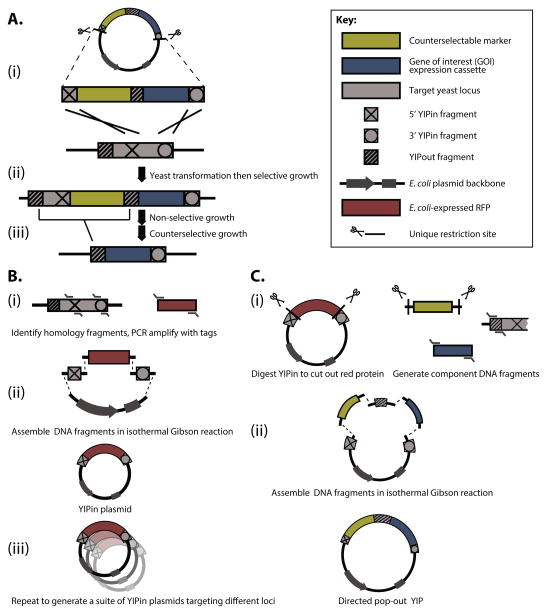
Figure 1. Process for integration and construction of directed pop-out YIPs.
A. Chromosomal integration of an expression cassette for a gene of interest (GOI) with a directed pop-out YIP. (i) The directed pop-out YIP containing a counterselectable marker and a GOI expression cassette is linearized by digestion. (ii) The integrated strain is grown overnight in non-selective media and then plated on solid counterselective media. (iii) Recombination between the designed tandem repeat (the YIPout region shown as a striped box) leads to the excision of the counterselectable marker leaving only the GOI expression cassette at the integrated locus. B. Assembly of a YIPin plasmid targeting a new locus. (i) YIPin homology regions are identified and PCR amplified from the yeast chromosome with primers bearing 5′ overhangs with designated tags for in vitro DNA assembly. (ii) The YIPin fragments are assembled with an E. coli plasmid fragment and the E. coli-expressed RFP construct to yield a YIPin plasmid. (iii) Repeating the YIPin assembly process for multiple loci yields a suite of YIPin plasmids that allows for rapid construction of directed pop-out YIPs capable of integrating into different loci. C. Assembly of a directed pop-out YIP. (i) A YIPin plasmid is digested to cut out the RFP gene; the counterselectable marker, GOI expression cassette, and YIPout fragment are generated with designated tags for in vitro DNA assembly. (ii) The fragments are assembled in a Gibson isothermal reaction to yield the directed pop-out YIP.