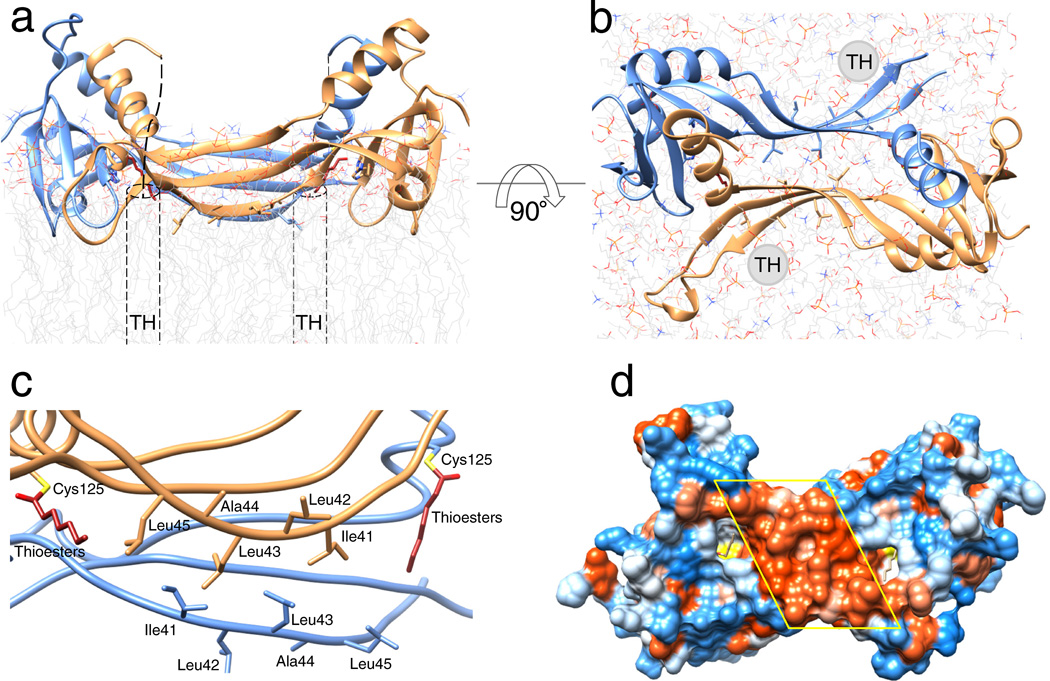
Figure 6. Phospholipid membrane topology of the HRASL3/LRAT chimera.
(a) and (b) Ribbon representations of the chimeric enzyme positioned at the lipid membrane viewed parallel and perpendicular to the membrane plane, respectively. Dashed cylinders represent putative positions of the C-terminal transmembrane α-helices (TM) absent in the crystallized protein. The overall topology was inferred from the parallel orientation of the acylated active sites and hydrophobicity of the protein/lipid interface. (c) Hydrophobic portion of the LRAT-specific domain with selected non-polar residues proposed to be involved in the lipid membrane interaction. (d) Hydrophobicity of the proposed membrane interaction surface. The molecular surface is colored according to the relative hydrophobicity of the side chains. Blue color corresponds to polar, whereas red indicates hydrophobic residues. The central hydrophobic patch corresponds to the membrane interacting region formed by residues indicated in panel c.