Abstract
Study of negligibly senescent animals may provide clues that lead to better understanding of the cardiac aging process. To elucidate mechanisms of successful cardiac aging, we investigated age-related changes in proteasome activity, oxidative protein damage and expression of heat shock proteins, inflammatory factors, and mitochondrial complexes in the heart of the ocean quahog Arctica islandica, the longest-lived noncolonial animal (maximum life span potential: 508 years). We found that in the heart of A. islandica the level of oxidatively damaged proteins did not change significantly up to 120 years of age. No significant aging-induced changes were observed in caspase-like and trypsin-like proteasome activity. Chymotrypsin-like proteasome activity showed a significant early-life decline, then it remained stable for up to 182 years. No significant relationship was observed between the extent of protein ubiquitination and age. In the heart of A. islandica, an early-life decline in expression of HSP90 and five mitochondrial electron transport chain complexes was observed. We found significant age-related increases in the expression of three cytokine-like mediators (interleukin-6, interleukin-1β, and tumor necrosis factor-α) in the heart of A. islandica. Collectively, in extremely long-lived molluscs, maintenance of protein homeostasis likely contributes to the preservation of cardiac function. Our data also support the concept that low-grade chronic inflammation in the cardiovascular system is a universal feature of the aging process, which is also manifest in invertebrates.
Key Words: Extreme longevity, Mollusc, Invertebrate, Model system, Cardiac.
Age is a major risk factor for cardiovascular disease, which remains the leading cause of morbidity and mortality of older Americans. Despite recent advances in the biology of aging, the factors determining successful cardiovascular aging are still not completely understood (1,2). Life span in the animal kingdom ranges more than 10,000-fold, and comparative studies on long-lived, successfully aging animals can elucidate key cellular mechanisms that may contribute importantly to successful cardiovascular aging (3,4). We have initiated a series of studies in long-living species to test predictions of major theories of aging and to elucidate key mechanisms for delaying cardiovascular aging (3–7).
In this study, we introduce the ocean quahog, Arctica islandica (Linnaeus, 1767), the longest-lived noncolonial animal known to science (8), as a study organism to cardiovascular aging research. The known maximum life span potential for this infaunal bivalve mollusc found in the shelf seas of the North Atlantic currently stands at 508 years (9) (Table 1). This extraordinarily long-lived species has recently piqued the interest of biogerontologists (10–14) because it may possess exceptional biological properties that defend against the vagaries of aging. Recent studies have characterized several aspects of A. islandica physiology (10,15,16) and used this novel invertebrate model organism to test predictions of the oxidative stress hypothesis of aging (12,17,18), to evaluate novel aspects of allometry of life span (11), and to elucidate the role of a multistress resistance phenotype in longevity (13). This study investigates age-related changes in the heart of this molluscan model of extreme longevity, A. islandica, the first study to undertake such an approach.
Table 1.
Chronological Age, Maximum Reported Life Span, and Life History Characteristics of the Population of Arctica islandica Sampled for This Study
| Species | Maximum Species Life Span (y) | Oldest Sampled (y) | Youngest Sampled (y) | Maximum Size (mm) | Growth Rate (K [VBGF]) | Mortality Rate (Z) | Age at Maturity (y) | Lifestyle | References |
|---|---|---|---|---|---|---|---|---|---|
| Artica islandica | 508 | 182 | 10 | 93.7 | 0.03 | 0.02 | 7 | Infaunal burrower | (57) |
The typical bivalve heart comprises a median ventricle that communicates with a pair of lateral equisized auricles through separate openings guarded by valves (19,20). The contractile machinery, the electrical properties of the myocytes, the role of mitochondria in energy supply, and the role of antioxidant and repair pathways in cellular homeostasis show remarkable similarities with the mammalian heart. Interestingly, in bivalves the heart is believed to serve two functions due to its close association with the gut: firstly to pump hemolymph around the circulatory system and secondly to facilitate the movement of fecal material through the gut running through the heart (19,20). During its life span spanning five centuries, the heart of A. islandica can beat more than 1.5 billion times. Yet, there are no studies extant investigating cardiac aging in this extraordinary animal model of successful aging. This study was designed to investigate whether age-related changes in the cardiac biology of mammals remain faithful in the heart of A. islandica and to facilitate our understanding of how this animal maintains its cardiac function throughout its exceptional life span.
In mammals, the progressive accumulation of oxidatively modified proteins is an important feature of aging (21,22), which has been implicated in the etiology or progression of a range of age-related disorders and diseases and serve as a reliable biomarker of aging (23,24). An increase in carbonyl content of proteins with age or with failing heart function has been documented in the mammalian heart (25–30). Here we seek to understand if accrual of protein oxidation with age is present in the heart of A. islandica.
In mammals, oxidatively modified proteins are not repaired and have to be removed by proteolytic degradation. Degradation of altered proteins is mostly accomplished by the evolutionarily conserved ubiquitin–proteasome system. Misfolded proteins are tagged for degradation with a small protein (ubiquitin) allowing the proteasome to degrade the tagged protein. In mammals, proteasome activity, critical for the maintenance of cellular viability, is demonstrated to decrease with age and believed to be a contributing factor to cardiac dysfunction during aging (30–34). The age-related failure of the proteasome system to deal with altered proteins likely leads to progressive accumulation of aggregates of ubiquitinated proteins with age (35). Despite its potential importance in regulation of life span, there are no studies extant investigating age-related changes in the ubiquitin–proteasome system in molluscan models of extreme longevity.
The heat shock proteins (HSPs) are a class of molecular chaperones characterized by their ability to modulate the structure and protect the correct folding of other proteins. HSPs have been demonstrated to regulate both stress resistance and life span in model organisms (36). In mammalian tissues, including the heart, expression of multiple HSPs decreases significantly with age (28), which is believed to play a role in age-related cardiac dysfunction. In one of the few investigations in clams, Ivanina and coworker reported an age-related decrease in the expression of HSP90 and HSP60 in Mercenaria Mercenaria (37). Presently no information is available on age-related changes in HSPs in A. islandica. It is believed that in many mammalian species (eg, long-lived bats), efficient maintenance of protein homeostasis may contribute to their unusually long life span (38). This concept is also supported by data obtained from invertebrate model organisms (36). To test the hypothesis that exceptional longevity of A. islandica is associated with an ability to maintain protein homeostasis over a long period of time, we assessed age-related changes in activities of proteasome activities, cellular accumulation of ubiquitinated proteins, and expression of HSPs in the heart tissue.
Mitochondrial decay has been postulated to be an important mechanism underlying part of the aging process (39). Indeed, age-related dysregulation of mitochondrial biogenesis and consequent decline in mitochondrial content have been documented in multiple vertebrate species in various tissues, including the myocardium (reviewed recently in Ref. 1). Decline in mitochondrial number and/or mitochondrial dysfunction may lead to enhanced ROS generation and cellular energy deficits, compromising vital ATP-dependent cellular processes, including detoxification pathways, repair systems, DNA replication, and transport mechanisms. In vertebrates, the heart is thought to be especially sensitive to age-related dysregulation of mitochondrial biogenesis and mitochondrial dysfunction due to the dependency of cardiac myocytes on beta-oxidation of fatty acids for energy and the postmitotic nature of cardiac tissue (which allows for greater accumulation of mitochondrial mutations and deletions) (1). Thus, the maintenance of mitochondrial content is likely critical to preservation of myocardial function in extremely long-lived animals. In this study, age-related changes in the cardiac expression of five complexes that make up the electron transport chain in A. islandica are, therefore, investigated.
In mammals, aging is associated with a significant proinflammatory shift in the cytokine expression profile in the cardiovascular system (40–49), which have been linked to the development of various age-related pathologies, including atherosclerosis and heart failure (50) (reviewed recently in Ref. 2). Molluscs have an innate immune system, and although lacking the adaptive immune systems of vertebrates, do share common signaling processes, for example, various cytokines, employed by vertebrates in eliminating pathogens and parasites (51). There is a significant body of literature suggesting the existence of cytokines in invertebrates and their role in invertebrate immunity (51–55); yet, age-related changes in the expression profile of molluscan cytokine-like mediators have not been documented. We, therefore, investigate age-related changes in heart tissue of A. islandica of three cytokines (tumor necrosis factor-α [TNF-α], interleukin-6 [IL-6], and interleukin-1β [IL-1β]) associated with age-related heart disease in mammals and documented to occur in bivalves.
Methods
Clam Collection and Maintenance
Specimens of the extremely long-lived ocean quahog (A. islandica) used in this study were collected from a site at 20–25 m depth at the mouth of Belfast Lough (54′842.10°N 58′35.25°W) in April 2010, using a customized rigid-toothed dredge, 1 m wide with a 25mm mesh, deployed from the RV “Prince Madog.” Belfast Lough is a fully marine inshore body of water that experiences annual seawater temperature ranges of between 6°C and 15°C (56). Ridgway and coworker undertook a comprehensive demographic study of the A. islandica population in Belfast Lough, documenting maximum life spans of 220 years (57). Maximum species life span and physiological characteristics for the species at this location are presented in Table 1. Although documented to live more than 500 years, in some parts of its range, the population sampled for this study have a documented longevity of 220 years (57).
The clams were transported to the aquaria at the School of Ocean Sciences, Bangor University, UK, where they were held at ambient temperature in 500L tanks for 3 months prior to the studies. On the day of the experiments, the quahogs were dissected. Heart tissue was isolated using microsurgery instruments and a stereo operating microscope and frozen in liquid nitrogen and stored at −80°C until shipping. When required for subsequent biochemical analysis, samples were shipped in dry ice to the Reynolds Oklahoma Center on Aging at the University of Oklahoma Health Sciences Center, Oklahoma City, where all subsequent biochemical analysis was undertaken.
Determination of Individual Age
Individual age of the clams used was determined from internal shell growth increments through sclerochronological analysis as previously described (18).
Analysis of Age-Related Changes in Protein Carbonylation in the Molluscan Heart
Protein carbonyl content was assessed in the heart tissue of A. islandica using the OxiSelect Protein Carbonyl ELISA Kit (Cell Biolabs Inc., San Diego, CA), according to the manufacturer’s guidelines. In brief, a standard curve of carbonylated bovine serum albumin (BSA) was constructed by mixing oxidized BSA and reduced BSA in predetermined ratios. Heart tissues were homogenized and adjusted to 10 µg protein/mL for each sample. A 100 µL volume of 10 µg/mL protein samples were used. Samples and reduced/oxidized BSA standards were then incubated overnight at 37°C in a 96-well protein binding plate and afterward washed thrice with PBS. Following the wash step, 100 µL of 2,4-dinitrophenylhydrazine working solution was added and the plate incubated for 45 minutes in darkness at room temperature. The wells were then washed with 250 µL of a 1:1 solution of PBS and ethanol incubated on an orbital shaker for 5 minutes, and the wash steps were repeated five times. After the last wash, wells were washed with PBS, and then 200 µL of blocking solution was applied to each well and incubated for a further 2 hours at room temperature on an orbital shaker. The wells were then washed again with 250 µL wash buffer, and then 100 µL of anti-2,4-dinitrophenyl antibody was added before a further 1-hour incubation at room temperature on the orbital shaker, following which wells were again washed thrice with the wash buffer. Following these washes, 100 µL of diluted horseradish peroxidase–conjugated secondary antibody was added to all wells and incubated for another 1 hour at room temperature on an orbital shaker and the plate washed a further five times with a wash buffer. The substrate solution (100 µL) was then added to all wells, including blank wells, and the plate incubated at room temperature on an orbital shaker for 30 minutes following which the reaction was stopped with the addition of 100 µL stop solution to each well. Absorbance was read immediately on a Infinite M200 plate reader (Tecan, Research Triangle Park, NC) at 450 nm with the fully reduced BSA standard as absorbance blank.
Analysis of Age-Related Changes in Proteasome Activity in the Molluscan Heart
To compare protein recycling activities in heart of A. islandica, we assessed three types of protease activities associated with the proteasome complex in heart samples using the Proteasome-Glo Chymotrypsin-Like, Trypsin-Like, and Caspase-Like Assays (Promega, Madison, WI), according to the manufacturer’s guidelines. Briefly, luciferin detection reagent was mixed with Proteasome-Glo cell-based buffer, and then appropriate Proteasome-Glo peptide substrates for the chymotrypsin-like, trypsin-like, and caspase-like activities were added. For Proteasome-Glo trypsin-like assay, only the inhibitors 1 and 2 were added. The mixture was incubated at room temperature for 30 minutes, after which 50 µL of Proteasome-Glo cell-based reagent was added to 50 µL of heart supernatant samples. Reaction samples were mixed and incubated for a minimum of 10 minutes, and luminescence was measured on Infinite M200 plate reader.
Analysis of Age-Related Changes in Protein Ubiquitination in the Molluscan Heart
Protein ubiquitination was assessed by UbiQapture Western Blot (Enzo Life Sciences, Farmingdale, NY) according to the manufacturer guidelines. Briefly, the control ubiquitinylated-protein lysate (5 μL) was diluted with 95 μL of PBS at 4°C in a clean 1.5 mL Eppendorf tube (100 μL final volume) to prepare the control binding solution. This diluted sample contained 25 μg total protein at a concentration of 250 μg/mL. Using 25 μg total protein content as a starting point, the lysate samples were prepared for ubiquitin enrichment, in clean 1.5-mL Eppendorf tubes, by dilution of stock lysate solution in appropriate volumes of PBS to give a final diluted sample binding solution volume of 100 μL (protein concentration, 250 μg/mL). Starting material samples for subsequent analysis were prepared by addition of 25 μL 5× sodium dodecyl sulfate–polyacrylamide gel electrophoresis gels loading buffer to each 100 μL starting material lysate solution, followed by heating to 95°C for 10 minutes and stored at −20°C until required. The UbiQapture-Q matrix was suspended by gently inverting the tube several times, and then 40 µL volume was aliquoted into a fresh tube for each of the samples to be analyzed. PBS (200 μL) was added to each tube, mixed gently by inversion, and centrifuged for 10 seconds at 5,000g; then PBS buffer was removed carefully without disturbing the matrix pellet. Sample or control binding solution (100 μL) was added to tube containing washed UbiQapture-Q matrix, resuspended gently by inversion, and then incubated at 4°C on a horizontal rotor mixer for a minimum of 4 hours. Subsequently, samples were centrifuged for 15–30 seconds at a speed of 5,000g to collect the UbiQapture-Q matrix. The supernatant (unbound fraction) was aspirated carefully (approximately 100 μL). Samples of unbound fraction were prepared for subsequent analysis by addition of 25 μL 5× sodium dodecyl sulfate–polyacrylamide gel electrophoresis gel loading buffer followed by heating to 95°C for 10 minutes. The matrix was washed with 200 μL PBS. Samples were centrifuged for 30 seconds at 5,000g to collect the UbiQapture-Q matrix. The supernatant was removed carefully (wash fraction). Wash with PBS was repeated once more. Ubiquitin–protein conjugates were eluted by addition of 100 μL PBS and 25 μL 5× sodium dodecyl sulfate–polyacrylamide gel electrophoresis gel loading buffer to each matrix containing sample (elution fraction). Quench was performed by mixing at room temperature for 5 minutes, followed by heating to 95°C for 10 minutes. The samples were analyzed by Western blotting.
Analysis of Age-Related Changes in HSP Expression in the Molluscan Heart
Determination of HSP in the heart tissue was performed using the MultiBead HSP/Chaperone 8-plex kit for flow cytometry (Enzo Life Sciences, Farmingdale, NY) according to the manufacturer’s guidelines. Fold changes in the expression of HSP90, HSP70, HSP60, and HSP40 over four age groups were analyzed. The assay utilizes monoclonal antibodies or antigen affinity purified polyclonal antibodies covalently coupled to latex beads. Briefly, 100 µL of the assay buffer was pipetted to the bottom of each well of the 96-well plate. The buffer was aspirated by vacuum manifold, and the plate was tapped firmly on a lint free paper towel to remove residual buffer. Then 50 µL of the assay buffer was added to each well followed by 50 µL of standard, 50 µL of heart samples, and 50 µL of diluted beads. The plate was sealed and incubated for 1 hour at room temperature, shaken at 700 rpm. Next, the plate was aspirated on vacuum manifold and washed thrice by adding 200 µL of wash buffer. Following the final wash, the plate was removed from the vacuum manifold and tapped to remove residual buffer. Then, 100 µL of the diluted (1×) antibody mixture was added to every well; the plates were sealed and incubated for 1 hour at room temperature on a shaker, and washed thrice; finally, 100 µL of the diluted (1×) MultiBead-streptavidin-PE conjugate was added to each well. The plate was again sealed, incubated for 30 minutes on a shaker at room temperature, and rewashed thrice as above. Finally, 200 µL of wash buffer was added to each well, and the beads vigorously pipetted up and down. The samples were evaluated by flow cytometry (GUAVA 8HT, Merck Millipore, Billerica, MA).
Analysis of Age-Related Changes in the Expression of Mitochondrial ETC Complexes in the Molluscan Heart
To analyze age-related changes in the expression of mitochondrial ETC complexes in heart tissue of A. islandica, the BeadPlex Human Mitochondrial Toxicity: Oxidative Phosphorylation Panel (Millipore, Merck KGaA, Darmstadt, Germany) was used, according to the manufacturer’s guidelines. Quantitative determination of the following ETC complexes was performed: complex I (NADH-ubiquinone oxidoreductase), complex II (succinate ubiquinone oxidoreductase), complex III (ubiquinone cytochrome C oxidoreductase), complex IV (cytochrome C oxidase), and complex V (ATP synthase). A 96-well filter plate was prewet with 100 µL of 1× assay diluent and incubated for 30 seconds, and the liquid was removed by vacuum filtration. To the plate, 1× capture Bead Mix was added, then the heart samples and controls were added, followed by 1× detection antibody mix; the plate was incubated for 3 hours in darkness at room temperature on an orbital plate shaker at 700 rpm. The plate was then washed thrice with 100 µL wash buffer and 50 μL 1× streptavidin-PE added to each well, followed by a 30-minute incubation at room temperature on an orbital shaker. The plate was then again washed thrice with 100 µL 1× wash buffer and beads were resuspended with 120 µL 1× wash buffer before being shaken for 5 minutes in darkness at room temperature on an orbital shaker; then the plate was read on Luminex Bio-Plex 200, System.
Analysis of Age-Related Changes in Cytokine-Like Factors in the Molluscan Heart
The expression of IL-6-like, IL-1β-like, and TNF-α-like cytokines (which are important biomarkers of aging in the mammalian heart) in cardiac homogenates was analyzed using the Multiplex MAP Cytokine/Chemokine Magnetic Bead Panel (Millipore, Merck KGaA, Darmstadt, Germany). Briefly, 200 µL of wash buffer was added to each well of a 96-well plate that was sealed and mixed on a plate shaker for 10 minutes at room temperature. The wash buffer was then decanted from all wells; 25 µL of each standard, control, and sample was added, and 25 µL of assay buffer was added to the entire plate. Twenty-five microliters of premixed beads was then added to each well, and the plate was resealed and incubated with agitation overnight at 4°C. Next, well contents were gently removed with a hand-held magnet; the plate was washed twice and then 25 µL of detection antibodies added to each well. The plate was then again sealed and incubated with agitation for 1 hour at room temperature and 25 µL of streptavidin–phycoerythrin added to each well containing the 25 µL of detection antibodies. After which the plate was again sealed and incubated with agitation in darkness for 30 minutes at room temperature. Again well contents were gently removed using the hand-held magnet and the wells washed twice before 150 µL of Sheath Fluid was added to each well and beads resuspended on a plate shaker for 5 minutes. The plate was then run on Luminex Bio-Plex 200.
Protein Concentration Measurements
For normalization purposes, the protein content of each sample was determined by a spectrophotometric quantitative method using BCA reagent (Thermo Scientific, Rockford, IL).
Data Analysis
Statistical analyses of data were performed by regression analysis or analysis of variance (ANOVA) followed by the Tukey post hoc test, as appropriate. To investigate age-related changes in the degree of protein carbonylation, protein ubiquitination, and proteosomal activity, we investigated the linear relationship between each parameter and age of the animal from which the heart tissue was derived through regression analysis. To investigate changes in the expression of HSPs, mitochondrial ETC complexes, and cytokine-like factors, fold changes in the expression of each of the parameters over four age groups (20–40 years, 41–80 years, 80–120 years, and >120 years) were analyzed. We initially assessed the various outcomes at baseline across these four age groups using Kruskal–Wallis test or ANOVA (if normality assumption was fulfilled) followed by appropriate pairwise comparisons. A value of p < .05 was considered statistically significant. Data are expressed as means ± S E M, unless otherwise indicated.
Results
Analysis of the shells indicated that the oldest animal sampled was 182 years old (Table 1). Although the species has been documented to live in excess of 500 years, such extreme estimates of life span appear to be from one specific location with maximum life spans of around 200–250 years more common for the species in the North Atlantic, suggesting that the species in the sampled location is not a short-lived population compared with the Baltic Sea populations where this species lives for 60 years. The animals were assigned one of four age groups (20–40 years, 41–80 years, 80–120 years, and >120 years) to investigate age-related changes in protein expression in the heart tissue.
Age-Related Changes in Protein Carbonylation in the Molluscan Heart
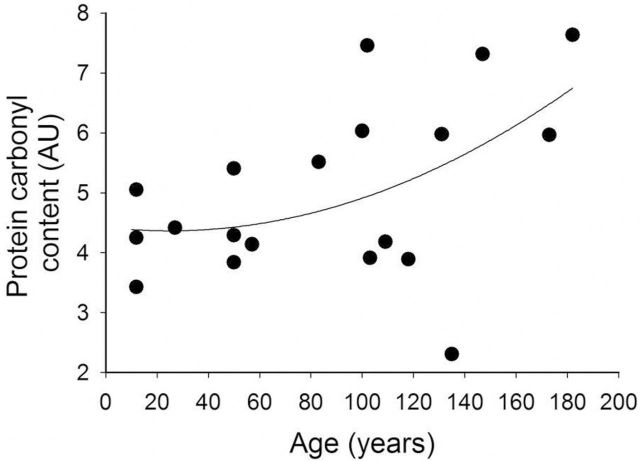
Protein carbonyl content in the cardiac muscle did not change significantly up to 120 years of age, whereas in older animals a gradual increase was observed (Figure 1). Over the entire age range investigated, the positive association between age and cardiac protein carbonyl content was significant (n = 18, F = 4.630; p = .046, r 2 = .168) (Figure 1).
Figure 1.
The relationship between the protein carbonyl content (AU) of the heart tissue and age of Arctica islandica. Regression analysis: n = 18, F = 4.630; p = .046, r 2 = .168.
Age-Related Changes in Proteasome Activity in the Molluscan Heart
To test the hypothesis that long-term maintenance of proteasome homeostasis may protect the cardiac muscle during aging and contribute to the exceptional longevity of A. islandica, we measured three types of peptidase activities of the 20S/26S proteasome. Although in the case of chymotrypsin-like activity (Figure 2A) we observed a significant positive association between the protease activity of cardiac muscle and age (n = 19, F = 48.864, p = 0 < .001, r 2 = .73), these changes manifested relatively early during the animal’s life, and then trypsin-like proteasome activity was maintained for a century. For trypsin-like proteasome activity (Figure 2B; n = 19, F = 3.235, p = .089, r 2 = .105) and caspase-like proteasome activity (Figure 2C; n = 19, F = .629, p = .438, r 2 = .001), there was no significant relationship with age.
Figure 2.
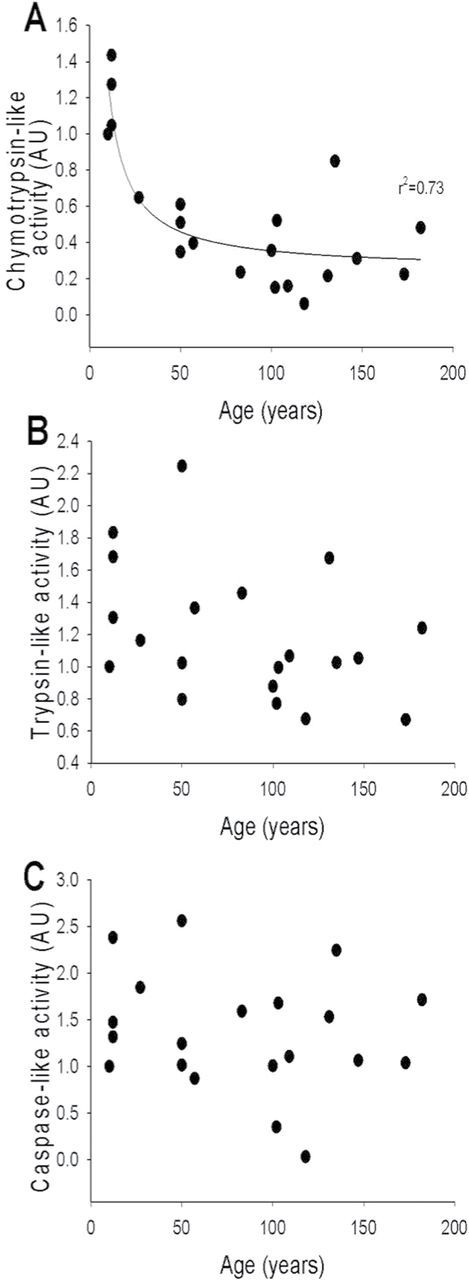
Proteasome activity (AU) in the hearts of Arctica islandica. Relationships between changes with age of chymotrypsin-like activity (A) (regression analysis: n = 19, F = 48.864, p = 0 < .001, r 2 = .73), trypsin-like activity (B) (regression analysis: n = 19, F = 3.235, p = .089, r 2 = .105), and caspase- like activity (C) (regression analysis: n = 19, F = 0.629, p = .438, r 2 = .001).
Age-Related Changes in Protein Ubiquitination
The extent of protein ubiquitination was determined through Western blot analysis (Figure 3A). The concentration of ubiquitinated proteins in the heart of A. islandica indicated no significant association with age (Figure 3B; n = 19, F = 0.542, p = .471, r 2 ≤ .001). No significant relationship was observed between protein carbonylation and the amount of ubiquitin-labeled protein (Figure 3C; n = 19, F = 0.0516, p = .823, r 2 ≤ .001). The relationship between the three investigated proteasome activities (chymotrypsin-like, trypsin-like, and caspase-like) and the amount of ubiquitin-labeled protein was also investigated, and again no significant association was observed (Figure 3D; chymotrypsin-like: n = 19, F = 0.0491, p = .492, r 2 ≤ .001; trypsin-like: n = 19, F = 1.467, p = .241, r 2 = .240; caspase-like: n = 19, F = 2.288, p = .153, r 2 ≤ .0687). Previously, Breusing and coworker (58) reported strong correlations between decline in proteasome activity and the increase in protein oxidation in liver and lung of aged rats. In this study, no correlation between protein damage and proteasome activity in the heart of A. islandica was observed (data not shown).
Figure 3.
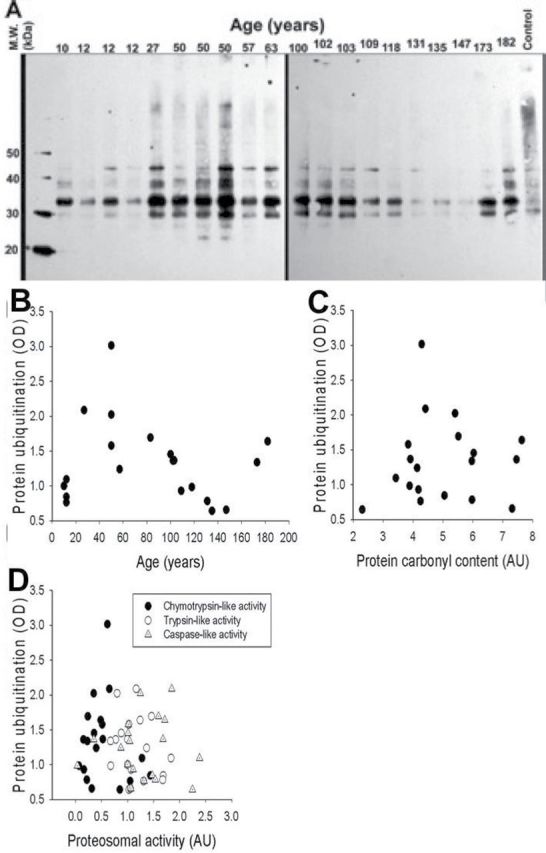
Protein ubiquitination was detected through Western blot analysis (A). Protein ubiquitination demonstrated no significant association with age (B; n = 19, F = 0.542, p = .471, r 2 ≤ .001). No significant relationship between protein carbonylation and protein ubiquitination was also observed (C; n = 19, F = 0.0516, p = .823, r 2 ≤ .001). There was also no significant relationships between protein ubiquitination and the three investigated proteasome activities (D; chymotrypsin-like: n = 19, F = 0.0491, p = .492, r 2 ≤ .001); trypsin-like: n = 19, F = 1.467, p = .241, r 2 = .240); caspase-like (n = 19, F = 2.288, p = .153, r 2 ≤ .0687).
Analysis of Age-Related Changes in HSP Expression in the Molluscan Heart
The investigation of variations of the expression of HSP with age focused on four specific HSPs: HSP40, HSP60, HSP70, and HSP90. All four HSPs investigated appeared to demonstrate consistent patterns with large initial decreases in activity (Figure 4A–D). However, due to high variability in the data, no significant age-related changes in the expression of HSP40, HSP60, and HSP70 were detected (Kruskal–Wallis Test: HSP40, H value = 4.58, p = .206; HSP60, H = 3.2, p = .362; HSP70, H = 2.45, p = .484). Significant changes were observed in the expression of HSP90 (Kruskal–Wallis Test: HSP40, H value = 8.86, p = .031); however, again high interanimal variability affected the outcome. Similar to the other three HSPs, there was a large initial decrease in expression of HSP90 from the first age quartile (20–40 years) to the second (41–80 years); however, this time a significant decrease in expression was detected. Further pairwise comparisons demonstrated that the two oldest age classes (80–120 and >120 years) were not significantly different from either of the two earlier age classes.
Figure 4.
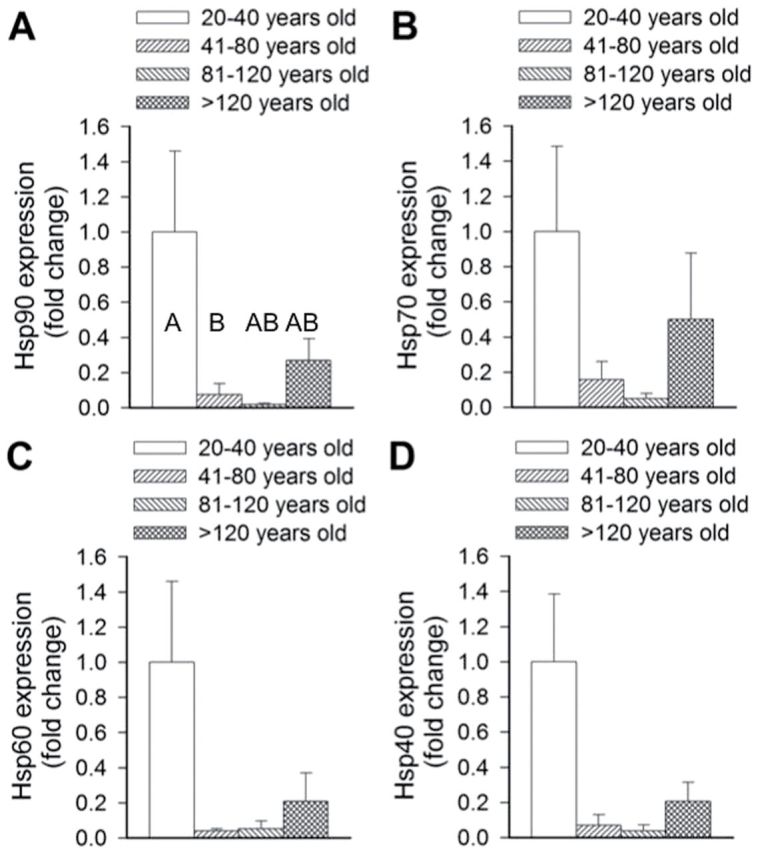
Age-associated changes in heat shock expression in Arctica islandica across four age quartiles: 20–40 years old, 41–80 years old, 81–120 years old, and >120 years old. Fold changes in expression from the youngest quartile of HSP90 (A), HSP70 (B), HSP60 (C), and HSP40 (D) were assessed in the heart tissue across the four age quartiles. Data are mean ± SEM. Different letters indicate significant differences between the age quartiles in (A), as indicated by pairwise comparisons. No significant differences were detected in Figure 4B–D.
Age-Related Changes in the Expression of Mitochondrial ETC Complexes in the Molluscan Heart
The investigation of variations of the expression of the five mitochondrial oxidative phosphorylation complexes (I–V) demonstrated consistent patterns with significant changes in expression with age (Kruskal–Wallis Test: complex I, H value = 9.36, p = .025; complex II, H = 14.37, p = .002; complex III, H value = 19.32, p < .001; complex IV, H value = 17.64, p = .001; complex V, H value = 11.66, p = .009). Further pairwise comparisons indicated consistent responses. With all complexes, significant reductions in activity between the first two age quartiles were observed. Although this reduction appeared to remain lower for the duration of the animals’ life span, no further significant reductions were observed. Although expression of complexes I, III, and IV in the third age quartile (81–120 years) appeared to be similarly low to those of the 41–80 and >120 years quartiles, they were not significantly lower or higher than any other age quartile, including the first. Similarly the expression of complex IV in the fourth age quartile (>120 years) was not significantly different than all preceding age quartiles (Figure 5A–E).
Figure 5.
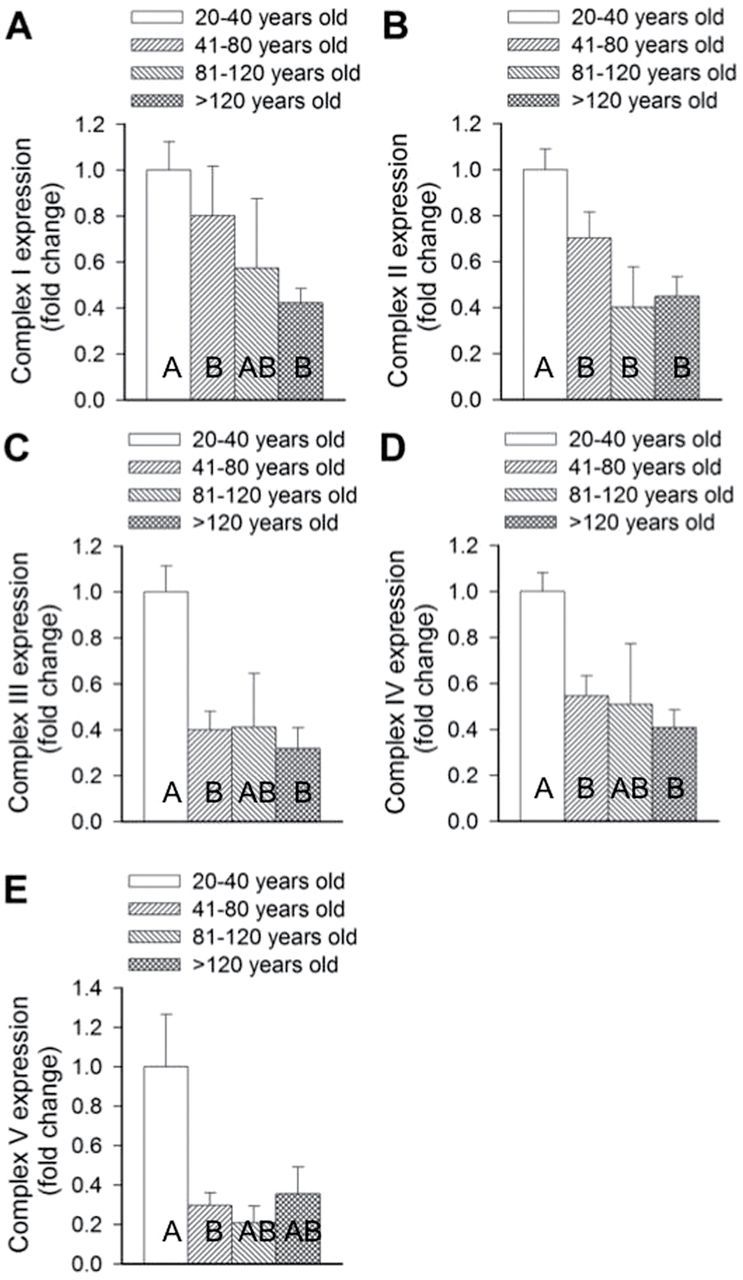
Age-associated changes in the expression of different complexes of the five mitochondrial oxidative phosphorylation complexes in Arctica islandica across four age quartiles: 20–40 years old, 41–80 years old, 81–120 years old, and >120 years old. Fold changes in expression from the youngest quartile of complexes I (A), II (B), III (C), IV (D), and V (E) were assessed in the heart tissue across the four age quartiles. Data are mean ± SEM. Different letters indicate significant differences between the age quartiles, as indicated by pairwise comparisons.
Age-Related Changes in Cytokine-Like Mediators in the Molluscan Heart
The concentration of IL-6-like, IL-1β-like, and TNF-α-like mediators demonstrated a very similar pattern with age (Figure 6A–C), with significant differences observed between the age quartiles for each cytokine (Kruskal–Wallis Test: IL-6, H value = 11.61, p = .009; IL-1β, H = 9.98, p = .019; TNF-α, H = 7.96, p = .047). For IL-1β, IL-6, and TNF-α, subsequent pairwise comparisons demonstrated the fold change in the expression of each cytokine-like mediator and demonstrated no change between the first two age classes but then a significant increase to the next age class (81–120 years). In the oldest age quartile (>120 years old), concentrations of IL-1β-like and TNF-α-like mediators were slightly lower than the preceding age class and were not significantly different from any of the age classes. In the case of IL-6-like cytokines, the oldest age quartile (>120 years old) was significantly higher than the lowest age quartile but was similarly not significantly different compared with the two other age quartiles (41–80 and 81–120 years).
Figure 6.

Age-associated changes in activity of cytokine-like molecules in Arctica islandica across four age quartiles: 20–40 years old, 41–80 years old, 81–120 years old, and >120 years old. Fold changes in concentration from the youngest quartile of interleukin-1β (A), interleukin-6 (B), and tumor necrosis factor-α (C) were assessed in the heart tissue across the four age quartiles. Data are mean ± SEM. Different letters indicate significant differences between the age quartiles, as indicated by pairwise comparisons, for example, in the case of IL-lβ, the third quartile is significantly elevated compared with the first two quartiles, but not the fourth.
Discussion
This study documented age-related changes in heart of A. islandica, the longest-lived noncolonial animal in the world. Sclerochronological analysis of the shells of sampled animals demonstrated that a large proportion of the species’ maximum life span at that location was investigated. The oldest animal sampled was documented to be 182 years old, with the population longevity at the sampled location (Belfast Lough, Northern Ireland, UK) estimated to be 220 years (57). Previous attempts to document age-related changes in the biology of longevous bivalves have failed to overcome this logistical barrier of sampling sufficiently aged animals, the oldest of the old, and here the ages sampled cover almost 85% of the known life span of that population. For example, Ivanina and coworker (37) reported changes in HSPs during the aging process of M. mercenaria, yet the oldest animals sampled were 4 years old, which depending on the population sampled covers 5%–10% of its maximum longevity and which has been estimated to vary between 50 and in excess of 100 years depending on location (59).
Recent gerontological research has documented that A. islandica exhibit enhanced resistance to multiple forms of stress compared with its shorter-lived relatives (12,13). Our previous findings demonstrating an association between longevity and organismal and cellular resistance to oxidative stress–induced injury and death in A. islandica is consistent with the oxidative stress hypothesis of aging (12). Our previous studies also support the premise that extreme longevity in A. islandica is associated with an attenuated cellular ROS production (12). In this study, we observe that the level of oxidatively damaged proteins in the cardiac muscle of A. islandica does not change significantly up to 120 years of age (Figure 1). Taken with our recent findings that protein carbonyl concentration in A. islandica gill tissue is reduced compared with tissues of shorter-lived hard clam, M. mercenaria, these data suggest that reduced ROS production in long-living bivalves is associated with lower levels of accumulated macromolecular damage (12), supporting the conclusion that cellular redox homeostasis may determine life span. Other researchers have also reported no age-associated increase in the protein carbonyl content of the gill and mantle tissue of A. islandica (60). Importantly, successfully aging mammals, such as bats (38), also tend to exhibit significantly lower protein oxidation (carbonylation) than shorter-lived species (22). Accrual of protein oxidation with age is also minimal in longer-lived muroid rodents (Peromyscus leucopus, maximum life span potential: 8.3 years) in contrast to the linear increase in short-lived Mus musculus (maximum life span potential: ~4 years) (22). In this study, very old animals exhibited a gradual age-associated increase in cardiac protein carbonyl content (Figure 1); however, the extent of these changes was lower than what has been reported in mammals.
In mammals, proteasome activity decreases during aging, which likely contributes to age-related pathophysiological alterations in the heart (30–34). Although proteasome activities have been investigated in short-lived and long-lived bivalves (12,17), this is the first study to focus on age-associated changes in proteasome activity in the molluscan heart. Importantly, we observed no significant age-associated changes in two types of peptidase activity of the 20S/26S proteasome (caspase-like and trypsin-like proteasome activity; Figure 2) in the heart of A. islandica. Although we did observe a significant decline in chymotrypsin-like proteasome activity in cardiac muscle of A. islandica, it manifested relatively early during the animal’s life, and then chymotrypsin-like proteasome activity was maintained for over a century (Figure 2). We also did not observe a significant relationship between the extent of protein ubiquitination and age (Figure 3), which is likely due to the low levels of damaged proteins in the heart of A. islandica during aging. The data in this study demonstrate for the first time that the ubiquitin–proteasome pathway is largely preserved in the heart of A. islandica during aging. It is expected that even centuries-old cardiac myocytes would maintain the turnover of key 26S proteasome substrates (transcription factors, cyclins, etc.) and prevent the accumulation of potentially toxic 20S proteasome substrates, such as oxidized proteins. The low levels of oxidatively damaged proteins and ubiquitinated proteins and the preserved proteasome activity in aged A. islandica are consistent with the hypothesis that long-term maintenance of proteasome homeostasis contributes to successful cardiac aging in this bivalve species.
In this study, we documented a consistent pattern of decline in four specific HSPs with age in the heart tissue; however, following statistical analysis, only the initial decrease in HSP90 was significant (Figure 4). In one of the few previous investigations into age-associated changes in HSPs, Ivanina and coworker (37) reported that these responses were HSP specific, with an age-related decrease in the expression of HSP90 and HSP60, and a concomitant increase in HSP70. However, the studies of Ivanina and coworker (37) had important limitations because only 5%–10% of the maximum life span of M. mercenaria was investigated. It is unclear whether the initial decrease in HSP90 expression is an indication of a general decline in molecular chaperones with age. Alternatively, a decrease in HSP90 in early life may reflect a developmental change unrelated to senescence. Interestingly, in other organisms, a wide variability in age-related changes of cytoplasmic HSPs has been documented, with increases (21), no changes (61), or decreases (28) reported. The HSP90 family, which comprises 1%–2% of all cellular proteins making it one of the most abundant proteins even in the absence of stress, acts downstream of the HSP70/HSP40-chaperone system and plays an important role in conformational protein regulation and cell signaling. Although data on the expression of these HSPs throughout the life span of A. islandica are especially important, environmental challenges will affect HSP70/90 gene regulation, in addition to the effects of aging. Therefore, investigators should be conservative in drawing conclusions from variations in HSP expressions between studies or between wild populations or species from different locations.
There is a wide range of studies both in invertebrate model organisms (62,63) and vertebrate animals (1,64–66) that implicate mitochondrial dysfunction and dysregulation of mitochondrial biogenesis as a causative factor in the cardiac aging process. Both the mammalian and the molluscan hearts have a relatively high metabolic demand compared with other tissues and are rich in mitochondria. The existing literature support the concept that aging-induced changes in mitochondrial biology, including dysregulation of mitochondrial biogenesis and a decline in cardiac mitochondrial content, are causally linked to the decline in cardiac performance with age in mammals (66). This study documents a consistent early-life decline in expression of all five ETC complexes, which potentially reflect a decline in mitochondrial content, in the cardiac muscle of A. islandica (Figure 5). It is possible that these mitochondrial changes in early life reflect a developmental change unrelated to senescence such as the early life decline in HSPs. A previous study documented a similar developmental change through the investigation of age-related changes in antioxidant parameters in A. islandica from 4 to 200 years (18). In the gill and mantle tissues, catalase, citrate synthase activity, and glutathione concentration declined rapidly within the first 25 years, covering the phase of rapid somatic growth and thereafter remained constant for >150 years. The similarity of the results in the two studies suggests that changes with age through developmental reasons rather than ageing may be a common observation in indeterminately growing animals with an initial phase of rapid somatic growth. Importantly, in the hearts of A. islandica, there were no further declines in mitochondrial ETC complexes for more than a century. This observation is consistent with the concept that preservation of mitochondrial integrity is an important factor in successful cardiac aging. Further studies are warranted to assess age-related changes in mitochondrial function and mitochondrial volume and density in A. islandica. It is important to note that the heart in mammals is largely composed of postmitotic cells; thus, mitochondria in the mammalian heart likely are more adversely affected by aging than other organs (67). In contrast, cells in the heart of A. islandica are likely capable of self-regeneration (associated with rejuvenation of the mitochondrial pool), a process which likely contributes to the preservation of mitochondrial integrity for centuries. In a previous study, Philipp and coworker (68) compared mitochondrial function of the longer-lived Laternula elliptica with Mya arenaria, which represent the same ecotype (benthic infaunal filter feeders) but differ in maximum life span, 36 and 13 years, respectively. They documented that the short-lived M. arenaria demonstrated a more pronounced decrease in mitochondrial function (respiration, respiratory control ratio, proton leak, and membrane potential) than its longer-lived relatives, and tissue redox state in L. elliptica remained stable throughout all ages, whereas it increased dramatically in aged M. arenaria. These results along with the results of this study are consistent with the concept that preservation of mitochondrial integrity is an important factor in successful aging in bivalves.
There is growing evidence that chronic low-grade inflammation, characterized by a proinflammatory shift in the profile of secreted cytokines, evolves during aging in the mammalian cardiovascular system (40–49). The innate immune system of molluscs is believed to share the common signaling molecules found in higher vertebrates, including cytokines, and there is strong evidence to believe these occur in molluscs. Our studies document significant age-related increases in the concentration of three cytokine-like mediators (IL-6, IL-1β, and TNF-α) in the heart of A. islandica (Figure 6), which support the hypothesis that low-grade chronic inflammation in the cardiovascular system is a universal feature of the aging process. In mammals, IL-6, IL-1β, and TNF-α are commonly associated with a proinflammatory response, which has been linked to increased oxidative stress, impaired mitochondrial function, increased apoptosis, and reduced myocyte contractile function. Importantly, IL-6, IL-1β, and TNF-α are also elevated in direct relation to deteriorating functional class of heart failure (50,69). Although the functional consequences of the observed age-related changes in inflammatory cytokine expression in the molluscan heart are unknown, the age-associated increase in proinflammatory cytokines presented in this study provides support for the phenomenon referred to as “inflamm-aging (70)” and demonstrates that molluscs exhibit similar proinflammatory responses in the cardiac muscle with age as mammals. Importantly, our studies support the concept that in successfully aging animals, age-related chronic low-grade inflammation in the cardiovascular system manifests much later in life than in shorter-living species.
Age-related changes in invertebrate immune functions have been documented in several species, some of them outside the usual model animals; for example, cellular immunosenescence has been reported in the cricket, Gryllus assimilis (71), and the fruit fly Drosophila melanogaster. Importantly, several studies report increased transcripts of immune response genes in D. melanogaster with age (72). These results and the present study strongly suggest that age-related immunosenescence (inflamm-aging) is a universal feature of the aging process. Despite the taxonomic distance between bivalves and mammals, their cardiovascular systems show remarkable similarities, with a well-developed hemal system consisting of the heart and vessels, which plays important physiological roles, including gas exchange, osmoregulation, nutrient distribution, waste elimination, and immune function. Here the heart has been the focus of study; however, equally important is the maintenance of the vasculature throughout the life span. As most age-related human diseases (including cardiovascular disease, stroke, and neurodegenerative diseases) involve some type of vascular/microvascular pathologies, future studies should, therefore, also investigate vascular aging in bivalve models of extreme longevity.
Limitations of the Study
This study has had to deal with two logistical problems that make analyzing the results more difficult but also may obscure or mask age-associated changes in cardiac biology. This study is unique in its investigation of age-associated changes of a wild population of a species; however, this brings its own challenges—aging in free-living animals varies between individuals and populations (73). Although controlled at the population level, longitudinal studies remain the desired approach even though the necessity to study wild-caught specimens of A. islandica brings the challenge of unavoidable individual heterogeneity. Through sampling a single location and population, we have tried to constrain this heterogeneity. However, an individual’s somatic “state” will be reflected in a wide range of phenotypic traits, such as body size or condition and immune response, which themselves often deteriorate with age, and these cannot be controlled and so adds intraspecific variability to the data. Additionally, in studies based on cross-sectional (as in this study) rather than longitudinal observations, there is often an influence of a phenomenon referred to as a “survivorship effect (74),” which can mask age-associated changes in the cardiac biology of the animals under study. Such “survivorship effects” may contribute to the apparent changes at advanced ages observed in this study, for example, in the expression of complex V and HSP90.
Because antibodies directed against molluscan proteins are not available, we chose to use antibodies directed against evolutionarily conserved human orthologs. In previous studies, antibodies directed against human cytokines were demonstrated to detect molluscan cytokine-like mediators (75–78). Further, invertebrate hemocytes have been induced to produce cytokine-like molecules in response to signals that elicit cytokine production in mammalian leukocytes, and a cytokine inhibitor, a human anti-TNF-α antibody, was shown to block cellular responses mediated by molluscan cytokines (75).
Conclusions
To our knowledge, this is the first study investigating age-related changes in the heart of this molluscan model of extreme longevity, A. islandica. Our findings support the concept that fundamental mechanisms of cardiac aging, including the role of inflammation, may be evolutionarily conserved. The documented similarities between age-related changes in mammalian and bivalve hearts provide justification for further investigations into the cardiac biology of this exceptionally long-lived species, especially for the evaluation of pathways involved in the protection of the proteome (eg, pathways involved in autophagy) in the longest-living noncolonial animal.
Funding
This work was supported by grants from the American Heart Association (to A.C., Z.U.), the National Center for Complementary and Alternative Medicine (R01-AT006526 to Z.U.); the National Institute on Aging (AG031085 to A.C., AG038747 to W.E.S.), the American Federation for Aging Research (to A.C.), the Oklahoma Center for the Advancement of Science and Technology (to A.C., Z.U., W.E.S.), Hungarian Scientific Research Fund (OTKA; K 108444 and the Nemzeti Fejlesztési Ügynökség (SROP-4.2.2.a-11/1/KONV-2012-0024 and -0017 to Z.U.), and the Ellison Medical Foundation (to W.E.S.)) . This work was supported by a National Institute on Aging/Biotechnology and Biological Sciences Research Council Partnering Award to Support Collaborative Research on the Biology of Aging (BB/H020535/1 to I.R.).
References
- 1. Dai DF, Rabinovitch PS, Ungvari Z. Mitochondria and cardiovascular aging. Circ Res. 2012;110:1109–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ungvari Z, Kaley G, de Cabo R, Sonntag WE, Csiszar A. Mechanisms of vascular aging: new perspectives. J Gerontol A Biol Sci Med Sci. 2010;65:1028–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ungvari Z, Krasnikov BF, Csiszar A, et al. Testing hypotheses of aging in long-lived mice of the genus Peromyscus: association between longevity and mitochondrial stress resistance, ROS detoxification pathways, and DNA repair efficiency. Age (Dordr). 2008;30:121–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ungvari Z, Buffenstein R, Austad SN, Podlutsky A, Kaley G, Csiszar A. Oxidative stress in vascular senescence: lessons from successfully aging species. Front Biosci. 2008;13:5056–5070. [DOI] [PubMed] [Google Scholar]
- 5. Labinskyy N, Csiszar A, Orosz Z, et al. Comparison of endothelial function, O2.- and H2O2 production, and vascular oxidative stress resistance between the longest-living rodent, the naked mole rat, and mice. Am J Physiol. 2006;291:H2698–H2704. [DOI] [PubMed] [Google Scholar]
- 6. Csiszar A, Labinskyy N, Zhao X, et al. Vascular superoxide and hydrogen peroxide production and oxidative stress resistance in two closely related rodent species with disparate longevity. Aging Cell. 2007;6:783–797. [DOI] [PubMed] [Google Scholar]
- 7. Csiszar A, Labinskyy N, Orosz Z, Xiangmin Z, Buffenstein R, Ungvari Z. Vascular aging in the longest-living rodent, the naked mole rat. Am J Physiol Heart Circ Physiol. 2007;293:H919–H927. [DOI] [PubMed] [Google Scholar]
- 8. Wanamaker AD, Jr, Heinemeier J, Scourse JD, et al. Very long-lived molluscs confirm 17th century AD tephra-based radiocarbon reservoir ages for north Icelandic shelf waters. Radiocarbon. 2008;50:1–14. [Google Scholar]
- 9. Butler PG, Wanamaker ADJ, Scourse JD, Richardson CA, Reynolds DJ.Variability of marine climate on the North Icelandic Shelf in a 1357-year proxy archive based on growth increments in the bivalve Arctica islandica. Palaeogeogr Palaeoclimatol Palaeoecol. 2013;373:141–151. [Google Scholar]
- 10. Ridgway ID, Richardson CA. Arctica islandica: the longest lived non colonial animal known to science. Rev Fish Biol. Fisher. 2010. 10.1007/s11160-11010-19171-11169 [Google Scholar]
- 11. Ridgway ID, Richardson CA, Austad SN. Maximum shell size, growth rate, and maturation age correlate with longevity in bivalve molluscs. J Gerontol A Biol Sci Med Sci. 2011;66:183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ungvari Z, Ridgway I, Philipp EE, et al. Extreme longevity is associated with increased resistance to oxidative stress in Arctica islandica, the longest-living non-colonial animal. J Gerontol A Biol Sci Med Sci. 2011;66:741–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ungvari Z, Sosnowska D, Mason JB, et al. Resistance to genotoxic stresses in Arctica islandica, the longest living noncolonial animal: is extreme longevity associated with a multistress resistance phenotype? J Gerontol A Biol Sci Med Sci. 2013;68:521–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ungvari Z, Philipp EE. Comparative gerontology–from mussels to man. J Gerontol A Biol Sci Med Sci. 2011;66:295–297. [DOI] [PubMed] [Google Scholar]
- 15. Abele D, Brey T, Philipp E. Bivalve models of aging and the determination of molluscan lifespans. Exp Gerontol. 2009;44:307–315. [DOI] [PubMed] [Google Scholar]
- 16. Philipp EE, Abele D. Masters of longevity: lessons from long-lived bivalves–a mini-review. Gerontology. 2010;56:55–65. [DOI] [PubMed] [Google Scholar]
- 17. Ungvari Z, Csiszar A, Sosnowska D, et al. Testing predictions of the oxidative stress hypothesis of aging using a novel invertebrate model of longevity: the giant clam (Tridacna derasa). J Gerontol A Biol Sci Med Sci. 2013;68:359–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Abele D, Strahl J, Brey T, Philipp EE. Imperceptible senescence: ageing in the ocean quahog Arctica islandica. Free Radic Res. 2008;42:474–480. [DOI] [PubMed] [Google Scholar]
- 19. Narain AS, Singh MP. An experimental investigation into the traversing of ventricle by gut in the unionid bivalve, Lamellidens corrianus. Experientia. 1974;30:1415–1416. [DOI] [PubMed] [Google Scholar]
- 20. Narain AS. A review of the structure of the heart of molluscs, particularly bivalves, in relation to cardiac function. J Moll Stud. 1976;42:46–62. [Google Scholar]
- 21. Cuervo AM, Dice JF. Age-related decline in chaperone-mediated autophagy. J Biol Chem. 2000;275:31505–31513. [DOI] [PubMed] [Google Scholar]
- 22. Shi Y, Pulliam DA, Liu Y, et al. Reduced mitochondrial ROS, enhanced antioxidant defense, and distinct age-related changes in oxidative damage in muscles of long-lived Peromyscus leucopus. Am J Physiol Regul Integr Comp Physiol. 2013;304:R343–R355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bhattacharya A, Leonard S, Tardif S, et al. Attenuation of liver insoluble protein carbonyls: indicator of a longevity determinant? Aging Cell. 2011;10:720–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chaudhuri AR, de Waal EM, Pierce A, Van Remmen H, Ward WF, Richardson A. Detection of protein carbonyls in aging liver tissue: a fluorescence-based proteomic approach. Mech Ageing Dev. 2006;127:849–861. [DOI] [PubMed] [Google Scholar]
- 25. Forster MJ, Sohal BH, Sohal RS. Reversible effects of long-term caloric restriction on protein oxidative damage. J Gerontol A Biol Sci Med Sci. 2000;55:B522–B529. [DOI] [PubMed] [Google Scholar]
- 26. Li Q, Wu S, Li SY, et al. Cardiac-specific overexpression of insulin-like growth factor 1 attenuates aging-associated cardiac diastolic contractile dysfunction and protein damage. Am J Physiol Heart Circ Physiol. 2007;292:H1398–H1403. [DOI] [PubMed] [Google Scholar]
- 27. Wu S, Li Q, Du M, Li SY, Ren J. Cardiac-specific overexpression of catalase prolongs lifespan and attenuates ageing-induced cardiomyocyte contractile dysfunction and protein damage. Clin Exp Pharmacol Physiol. 2007;34:81–87. [DOI] [PubMed] [Google Scholar]
- 28. Colotti C, Cavallini G, Vitale RL, et al. Effects of aging and anti-aging caloric restrictions on carbonyl and heat shock protein levels and expression. Biogerontology. 2005;6:397–406. [DOI] [PubMed] [Google Scholar]
- 29. Agarwal S, Sohal RS. Aging and proteolysis of oxidized proteins. Arch Biochem Biophys. 1994;309:24–28. [DOI] [PubMed] [Google Scholar]
- 30. Predmore JM, Wang P, Davis F, et al. Ubiquitin proteasome dysfunction in human hypertrophic and dilated cardiomyopathies. Circulation. 2010;121:997–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bulteau AL, Szweda LI, Friguet B. Age-dependent declines in proteasome activity in the heart. Arch Biochem Biophys. 2002;397:298–304. [DOI] [PubMed] [Google Scholar]
- 32. Grillari J, Grillari-Voglauer R, Jansen-Dürr P. Post-translational modification of cellular proteins by ubiquitin and ubiquitin-like molecules: role in cellular senescence and aging. Adv Exp Med Biol. 2010;694:172–196. [DOI] [PubMed] [Google Scholar]
- 33. Grillari J, Katinger H, Voglauer R. Aging and the ubiquitinome: traditional and non-traditional functions of ubiquitin in aging cells and tissues. Exp Gerontol. 2006;41:1067–1079. [DOI] [PubMed] [Google Scholar]
- 34. Li F, Zhang L, Craddock J, et al. Aging and dietary restriction effects on ubiquitination, sumoylation, and the proteasome in the heart. Mech Ageing Dev. 2008;129:515–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pérez VI, Buffenstein R, Masamsetti V, et al. Protein stability and resistance to oxidative stress are determinants of longevity in the longest-living rodent, the naked mole-rat. Proc Natl Acad Sci USA. 2009;106:3059–3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tower J. Heat shock proteins and Drosophila aging. Exp Gerontol. 2011;46:355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ivanina A, Sokolova I, Sukhotin A. Expression of heat shock proteins in aging bivalve mollusks. FASEB J. 2008;22:1239.1231(abstract). [Google Scholar]
- 38. Salmon AB, Leonard S, Masamsetti V, et al. The long lifespan of two bat species is correlated with resistance to protein oxidation and enhanced protein homeostasis. FASEB J. 2009;23:2317–2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Harman D. The biologic clock: the mitochondria? J Am Geriatr Soc. 1972;20:145–147. [DOI] [PubMed] [Google Scholar]
- 40. Csiszar A, Sosnowska D, Wang M, Lakatta EG, Sonntag WE, Ungvari Z. Age-associated proinflammatory secretory phenotype in vascular smooth muscle cells from the non-human primate Macaca mulatta: reversal by resveratrol treatment. J Gerontol A Biol Sci Med Sci. 2012;67:811–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang M, Monticone RE, Lakatta EG. Arterial aging: a journey into subclinical arterial disease. Curr Opin Nephrol Hypertens. 2010;19:201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bátkai S, Rajesh M, Mukhopadhyay P, et al. Decreased age-related cardiac dysfunction, myocardial nitrative stress, inflammatory gene expression, and apoptosis in mice lacking fatty acid amide hydrolase. Am J Physiol Heart Circ Physiol. 2007;293:H909–H918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ungvari Z, Orosz Z, Labinskyy N, et al. Increased mitochondrial H2O2 production promotes endothelial NF-kappaB activation in aged rat arteries. Am J Physiol Heart Circ Physiol. 2007;293:H37–H47. [DOI] [PubMed] [Google Scholar]
- 44. Csiszar A, Ungvari Z, Koller A, Edwards JG, Kaley G. Proinflammatory phenotype of coronary arteries promotes endothelial apoptosis in aging. Physiol Genomics. 2004;17:21–30. [DOI] [PubMed] [Google Scholar]
- 45. Csiszar A, Wang M, Lakatta EG, Ungvari Z. Inflammation and endothelial dysfunction during aging: role of NF-kappaB. J Appl Physiol (1985). 2008;105:1333–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pearson KJ, Baur JA, Lewis KN, et al. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8:157–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Csiszar A, Ungvari Z, Koller A, Edwards JG, Kaley G. Aging-induced proinflammatory shift in cytokine expression profile in coronary arteries. FASEB J. 2003;17:1183–1185. [DOI] [PubMed] [Google Scholar]
- 48. Wang M, Zhang J, Jiang LQ, et al. Proinflammatory profile within the grossly normal aged human aortic wall. Hypertension. 2007;50:219–227. [DOI] [PubMed] [Google Scholar]
- 49. Wang M, Zhang J, Telljohann R, et al. Chronic matrix metalloproteinase inhibition retards age-associated arterial proinflammation and increase in blood pressure. Hypertension. 2012;60:459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Deswal A, Petersen NJ, Feldman AM, Young JB, White BG, Mann DL. Cytokines and cytokine receptors in advanced heart failure: an analysis of the cytokine database from the Vesnarinone trial (VEST). Circulation. 2001;103:2055–2059. [DOI] [PubMed] [Google Scholar]
- 51. Ottaviani E. Molluscan immuno-recognition. ISJ. 2006;3:50–63. [Google Scholar]
- 52. Franchini A, Malagoli D, Ottaviani E. Cytokines and invertebrates: TGF-beta and PDGF. Curr Pharm Des. 2006;12:3025–3031. [DOI] [PubMed] [Google Scholar]
- 53. Ottaviani E, Franchini A, Malagoli D, Genedani S. Immunomodulation by recombinant human interleukin-8 and its signal transduction pathways in invertebrate hemocytes. Cell Mol Life Sci. 2000;57:506–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ottaviani E, Malagoli D, Franchini A. Invertebrate humoral factors: cytokines as mediators of cell survival. Prog Mol Subcell Biol. 2004;34:1–25. [DOI] [PubMed] [Google Scholar]
- 55. Malagoli D, Ottaviani E. Discrepant effects of mammalian factors on molluscan cell motility, chemotaxis and phagocytosis: divergent evolution or finely tuned contingency? Cell Biol Int. 2010;34:1091–1094. [DOI] [PubMed] [Google Scholar]
- 56. Evans GL, Hardman-Mountford NJ, Hartnoll RG, et al. Scientific Report No 2. Long- term environmental studies in the Irish Sea: a review. Defra Contract CDEP. 2003;84:311. [Google Scholar]
- 57. Ridgway ID, Richardson CA, Scourse JD, Butler PG, Reynolds DJ. The population structure and biology of the ocean quahog, Arctica islandica (Linnaeus, 1767), in Belfast Lough, Northern Ireland. J Mar Biol Assoc UK. 2012. 92:539–546. [Google Scholar]
- 58. Breusing N, Grune T. Regulation of proteasome-mediated protein degradation during oxidative stress and aging. Biol Chem. 2008;389:203–209. [DOI] [PubMed] [Google Scholar]
- 59. Ridgway ID, Richardson AC, Enos E, et al. New species longevity record for the Northern Quahog (=Hard Clam), Mercenaria mercenaria. J Shellfish Res. 2011;30:35–38. [Google Scholar]
- 60. Strahl J, Philipp EE, Brey T, Broeg K, Abele D. Physiological aging in the Icelandic population of the ocean quahog Arctica islandica. Aquatic Biol. 2007;1:77–84. [Google Scholar]
- 61. Wu B, Gu MJ, Heydari AR, Richardson A. The effect of age on the synthesis of two heat shock proteins in the hsp70 family. J Gerontol. 1993;48:B50–B56. [DOI] [PubMed] [Google Scholar]
- 62. Cho J, Hur JH, Walker DW. The role of mitochondria in Drosophila aging. Exp Gerontol. 2011;46:331–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sohal RS. Mitochondrial changes in the heart of Drosophila repleta, Wollaston with age. Exp Gerontol. 1970;5:213–216. [DOI] [PubMed] [Google Scholar]
- 64. Dai DF, Rabinovitch PS. Cardiac aging in mice and humans: the role of mitochondrial oxidative stress. Trends Cardiovasc Med. 2009;19:213–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Judge S, Leeuwenburgh C. Cardiac mitochondrial bioenergetics, oxidative stress, and aging. Am J Physiol Cell Physiol. 2007;292:C1983–C1992. [DOI] [PubMed] [Google Scholar]
- 66. Marzetti E, Csiszar A, Dutta D, Balagopal G, Calvani R, Leeuwenburgh C. Role of mitochondrial dysfunction and altered autophagy in cardiovascular aging and disease: from mechanisms to therapeutics. Am J Physiol Heart Circ Physiol. 2013;305:H459–H476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kwong LK, Sohal RS. Age-related changes in activities of mitochondrial electron transport complexes in various tissues of the mouse. Arch Biochem Biophys. 2000;373:16–22. [DOI] [PubMed] [Google Scholar]
- 68. Philipp E, Pörtner HO, Abele D. Mitochondrial ageing of a polar and a temperate mud clam. Mech Ageing Dev. 2005;126:610–619. [DOI] [PubMed] [Google Scholar]
- 69. Dinarello CA, Pomerantz BJ. Proinflammatory cytokines in heart disease. Blood Purif. 2001;19:314–321. [DOI] [PubMed] [Google Scholar]
- 70. Franceschi C, Bonafè M, Valensin S, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann NY Acad Sci. 2000;908:244–254. [DOI] [PubMed] [Google Scholar]
- 71. Park Y, Kim Y, Stanley D. Cellular immunosenescence in adult male crickets, Gryllus assimilis. Arch Insect Biochem Physiol. 2011;76:185–194. [DOI] [PubMed] [Google Scholar]
- 72. Pletcher SD, Macdonald SJ, Marguerie R, et al. Genome-wide transcript profiles in aging and calorically restricted Drosophila melanogaster. Curr Biol. 2002;12:712–723. [DOI] [PubMed] [Google Scholar]
- 73. Nussey DH, Pemberton JM, Pilkington JG, Blount JD. Life history correlates of oxidative damage in a free-living mammal population. Functional Ecology. 2009;4:809–814. [Google Scholar]
- 74. Newschaffer CJ, Bush TL, Hale WE. Aging and total cholesterol levels: cohort, period, and survivorship effects. Am J Epidemiol. 1992;136:23–34. [DOI] [PubMed] [Google Scholar]
- 75. Hughes TK, Jr, Smith EM, Barnett JA, Charles R, Stefano GB. LPS stimulated invertebrate hemocytes: a role for immunoreactive TNF and IL-1. Dev Comp Immunol. 1991;15:117–122. [DOI] [PubMed] [Google Scholar]
- 76. Hughes TK, Jr, Smith EM, Chin R, et al. Interaction of immunoactive monokines (interleukin 1 and tumor necrosis factor) in the bivalve mollusc Mytilus edulis. Proc Natl Acad Sci USA. 1990;87:4426–4429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Hughes TK, Jr, Smith EM, Leung MK, Stefano GB. Evidence for the conservation of an immunoreactive monokine network in invertebrates. Ann NY Acad Sci. 1992;650:74–80. [DOI] [PubMed] [Google Scholar]
- 78. Stefano GB, Smith EM, Hughes TK. Opioid induction of immunoreactive interleukin-1 in Mytilus edulis and human immunocytes: an interleukin-1-like substance in invertebrate neural tissue. J Neuroimmunol. 1991;32:29–34. [DOI] [PubMed] [Google Scholar]