Abstract
Background
Molecular characterization of breast cancer allows subtype-directed interventions. Estrogen receptor (ER) is the longest-established molecular marker.
Methods
We used six established population models with ER-specific input parameters on age-specific incidence, disease natural history, mammography characteristics, and treatment effects to quantify the impact of screening and adjuvant therapy on age-adjusted US breast cancer mortality by ER status from 1975 to 2000. Outcomes included stage-shifts and absolute and relative reductions in mortality; sensitivity analyses evaluated the impact of varying screening frequency or accuracy.
Results
In the year 2000, actual screening and adjuvant treatment reduced breast cancer mortality by a median of 17 per 100000 women (model range = 13–21) and 5 per 100000 women (model range = 3–6) for ER-positive and ER-negative cases, respectively, relative to no screening and no adjuvant treatment. For ER-positive cases, adjuvant treatment made a higher relative contribution to breast cancer mortality reduction than screening, whereas for ER-negative cases the relative contributions were similar for screening and adjuvant treatment. ER-negative cases were less likely to be screen-detected than ER-positive cases (35.1% vs 51.2%), but when screen-detected yielded a greater survival gain (five-year breast cancer survival = 35.6% vs 30.7%). Screening biennially would have captured a lower proportion of mortality reduction than annual screening for ER-negative vs ER-positive cases (model range = 80.2%–87.8% vs 85.7%–96.5%).
Conclusion
As advances in risk assessment facilitate identification of women with increased risk of ER-negative breast cancer, additional mortality reductions could be realized through more frequent targeted screening, provided these benefits are balanced against screening harms.
Breast cancer is a heterogeneous disease defined by molecular subtypes that predict treatment response and clinical outcomes (1–3). Identification of these molecular subtypes has become a critical step in breast cancer management as many new therapies have been developed and proven effective for specific subtypes (4–9). As the molecular characterization of breast cancer continues to influence individual patient management, the efficacy of current and emerging screening and treatment will need to be evaluated at the population level to understand how subtype-targeted strategies are affecting our ability to achieve cancer control objectives.
Estrogen receptor (ER) is the longest-established molecular marker in use for breast cancer treatment planning (10,11). Prior model-based analyses quantified the relative effects of screening mammography and adjuvant treatment at a population level (12–14); however, these effects have not been quantified by ER status. Moreover, even though the benefits of adjuvant therapy are known to vary by molecular subtypes, both in magnitude (11) and timing (15), these effects have yet to be assessed at a population level by ER status after accounting for the effect of screening. In general, simulation modeling provides a means to quantify the relative effects of cancer control interventions, especially when they occur simultaneously and interact, as in the case of screening and treatment. In this context, because the Surveillance, Epidemiology, and End-Results (SEER) program only began collecting ER status in 1990, modeling is also needed to leverage external sources of ER status to reconstruct population-level effects by ER status prior to 1990.
We present a comparative modeling analysis using six models to quantify the impact of screening and adjuvant therapy on US breast cancer mortality trends by ER status from 1975 to 2000. Our analysis illustrates the role of population cancer surveillance modeling to reinterpret trends in a molecular context. Our results are intended to contribute to ongoing discussions about how to more efficiently identify subgroups most likely to benefit from increased screening and/or targeted therapies (13,14,16–19) using knowledge about molecular subtypes.
Methods
Overview
Sponsored by the National Cancer Institute, the Cancer Intervention and Surveillance Modeling Network (CISNET) Breast Working Group has collaborated over the past 14 years to develop independent but comparable population models that reproduce incidence and mortality trends in the US population (12,20–25). Six groups participated in this study: Dana Farber (Model D), Erasmus Medical Center (Model E), Georgetown-Einstein (Model G-E), MD Anderson (Model M), Stanford (Model S), and Wisconsin-Harvard (Model W). The models have been described elsewhere (12,20–25); model updates are reported in the Supplementary Materials (available online). Briefly, all models share common inputs but differ in how they portray ductal carcinoma in situ (DCIS), screening effects, and impact of treatment. Despite these differences, in previous collaborations (12,16) all the models came to similar rankings of the relative contributions of screening and adjuvant treatment to observed decreases in deaths from breast cancer.
The current analysis incorporates modeling of molecular tumor subtypes and evaluates benefits under screening and adjuvant treatment regimens by molecular subtypes (hereafter, “treatment” refers to adjuvant treatment). ER status was chosen because it is the most common molecular marker and the only one with sufficient survival follow-up data for model validation. This research was classified as exempt by our Institutional Review Boards because it uses deidentified secondary data.
Intervention Scenarios
To estimate the effects of screening and treatment from 1975 to 2000 by ER status, each model independently simulated age-adjusted, ER-specific US breast cancer mortality trends under four intervention scenarios: 1) screening and treatment, 2) no screening and no treatment, 3) screening and no treatment, and 4) treatment and no screening. To estimate the effect of screening and treatment, scenarios 1 and 2 were compared; to estimate the effect of screening alone, scenarios 3 and 2 are compared; to estimate the effect of treatment alone, scenarios 4 and 2 are compared.
The models begin with estimates of ER-specific breast cancer incidence and mortality trends without screening and treatment for US birth cohorts and then overlay screening and treatment dissemination and survival improvements associated with treatment. Breast cancer is generally depicted as having a preclinical screening-detectable period and a symptomatic detection point. On the basis of mammography sensitivity, screening may detect tumors at an earlier stage than symptomatic detection, resulting in breast cancer mortality reduction. From 1975 to 2000, screening was done using plain film mammography; treatment focuses on adjuvant systemic therapy, including tamoxifen alone, chemotherapy alone, and both; surgery is included in all scenarios. Because hormonal therapies other than tamoxifen were not in widespread use until after 2000, they were not included. Death is modeled as a competing risk between breast cancer and other age-specific causes.
Model Inputs
Similar to previous work (12), each group relied on common inputs, including: background breast cancer age-period-cohort incidence (26), death from other causes (27), actual mammography (28,29) and treatment dissemination, (29–31) and age-, stage-, ER-specific treatment effectiveness (10,32).
For this study all models also required additional ER-specific inputs that were estimated using a statistical matching approach developed with Model S. This approach incorporated SEER survival data from 1975 through 1979 (which represents a period when screening and adjuvant treatment were not widespread) and data from 23000 women diagnosed with breast cancer between 1996 and 2009 provided by the Breast Cancer Surveillance Consortium (BCSC). The following ER-specific inputs were produced:
underlying breast cancer–specific survival curves in the absence of screening and treatment by ER status;
distribution of ER status for patients clinically detected in the absence of screening;
mean mammography detection thresholds by ER status, defined as the mean minimum tumor volume screen detectable;
mean sojourn times, defined as the mean duration between the time a tumor becomes screen detectable and the time of symptomatic detection, by ER status;
mean tumor volume doubling time (TVDT) by ER status.
All six models incorporated the underlying breast cancer–specific survival by ER status and the distribution of ER status at symptomatic detection; the other ER-specific inputs were used according to each group’s modeling requirements. An overview of common and ER-specific inputs used by each group is provided in Table 1. ER-specific inputs are provided in Supplementary Tables 1–5 (available online).
Table 1.
Overview of common inputs used by modeling group*
| Common inputs | Model | |||||
|---|---|---|---|---|---|---|
| D | E | G-E | M | S | W | |
| Those that do not vary by ER status | ||||||
| Background age-period-cohort incidence | + | + | + | + | + | |
| Death from other causes | + | + | + | + | + | + |
| Mammography dissemination | + | + | + | + | + | + |
| Treatment dissemination | + | + | + | + | + | + |
| Age, stage, and ER-specific treatment effectiveness | + | + | + | + | + | + |
| Those that vary by ER status | ||||||
| Baseline survival curves in absence of screening and treatment by ER status | + | + | + | + | + | + |
| Distribution of ER status in absence of screening and treatment | + | + | + | + | + | + |
| Mean mammography thresholds by ER status | + | + | ||||
| Mean sojourn times by ER status | + | + | + | + | ||
| Mean tumor volume doubling time by ER status | + | |||||
* ER = estrogen receptor.
Table 4.
Comparison between Model S and Breast Cancer Surveillance Consortium data for women diagnosed between 1996 and 2009
| Outcome | Model S | BCSC | ||
|---|---|---|---|---|
| ER+ cases | ER- cases | ER+ cases | ER- cases | |
| Probability of screen detection | 51.2% | 35.1% | 65.0% | 48.0% |
| Stage distribution among clinically detected cases *‡ | ||||
| Local | 49.9% | 51.0% | 47.2% | 47.0% |
| Regional | 42.9% | 42.1% | 48/3% | 47.5% |
| Distant | 7.2% | 6.8% | 4.5% | 5.4% |
| Stage distribution among interval cases †‡ | ||||
| Local | 57.5% | 55.5% | 61.9% | 60.6% |
| Regional | 36.8% | 37.9% | 35.6% | 34.7% |
| Distant | 5.7% | 6.7% | 2.5% | 4.7% |
| Stage distribution among screen-detected cases‡ | ||||
| Local | 77.8% | 72.9% | 79.5% | 74.5% |
| Regional | 20.7% | 24.8% | 19.9% | 24.3% |
| Distant | 1.5% | 2.3% | 0.5% | 1.1% |
* Cases detected in the absence of screening. BCSC = Breast Cancer Surveillance Consortium; ER = estrogen receptor.
† Cases missed on the screening exam and symptomatically detected within 12 months after the most recent screening exam.
‡ Distributions may not add up to 100% because of rounding errors.
Analysis
Each model estimated overall and ER-specific age-adjusted breast cancer mortality for women aged 30 to 79 years. ER-specific rates were calculated by multiplying the overall rate and the proportion of each subtype (ie, the number of cases of that subtype divided by all cases); in this manner, rates of both subtypes sum up to the overall breast cancer rate. Mortality reductions are portrayed as absolute reductions (ie, the difference between the rate predicted in the absence of screening and treatment and the rate predicted with one or both interventions), and percent-relative mortality reductions (ie, the percent corresponding to the difference between the rate predicted with no screening and no treatment and the rate predicted with either or both interventions divided by the former).
Since the benefits of screening are dependent on detecting the disease at an earlier, more curable stage, we quantified the breast cancer mortality reduction attributable to screening, in part, by quantifying the extent of stage shift by ER status. To perform this analysis, we compared the stage distribution and breast cancer survival between screening and no-screening scenarios. When noted, screen-detected patients who die of non–breast cancer causes in the lead-time were omitted to remove overdiagnosis bias when comparing screening and no-screening outcomes.
Sensitivity analysis was performed on a subset of inputs using two representative models that had large differences in outcomes (Models E and S). Screening frequency and mammographic detectability were varied from the base case, where actual dissemination of screening and ER-specific detectability parameters were used (Supplementary Table 1). When the screening frequency was varied, 100% screening compliance was assumed. Because ER-negative disease is harder to screen-detect than ER-positive disease, a sensitivity analysis was performed assuming that ER-negative disease is as screen detectable as ER-positive disease to quantify the effect of a difference in screen detectability by ER status on mortality.
Model Validation and Uncertainty
We used several approaches to validate the model and portray uncertainty. First we compared model predictions of overall and ER-specific age-adjusted incidence and mortality to actual SEER rates (see Supplementary Figures 1 and 2, available online, for incidence). Because SEER began collecting ER status in 1990, ER-specific SEER mortality rates were calculated using incidence-based mortality with imputation for unknown records, assuming known and unknown cases were distributed identically. We compared the model estimates of ER-specific mortality to actual SEER ER-specific rates from 1994 onward because earlier years had a large fraction of unknown cases. Next, we compared model-predicted ER-specific breast cancer survival curves with actual SEER survival curves in the presence of screening and treatment (Supplementary Figure 3, available online). Lastly, we compared model predictions with BSCS data that was not used to develop the model inputs, such as the probability of screen detection and stage distributions by ER status.
Each input parameter was sampled from a distribution, hereby incorporating individual-level variability. Moreover, using six models with different structures, assumptions, and variations in some input parameters provides implicit cross-validation, with the range of results from the models as a measure of uncertainty.
Results
The six models produced similar overall and ER-specific age-adjusted breast cancer mortality rates in comparison with SEER (Figure 1). Compared with the mortality rates expected if there had been no screening or treatment from 1975 to 2000, the models showed that absolute subtype-specific mortality was reduced in the year 2000 through dissemination of screening and treatment by a median of 17 per 100000 women (model range = 13–21) and five per 100000 women (model range = 3–6) for ER-positive and ER-negative cancers, respectively (Table 2).
Figure 1.
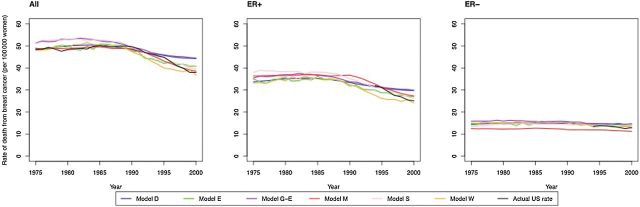
Breast cancer mortality rate comparison of six model predictions to actual US rates for patients ages 30 to 79 at diagnosis from 1975 to 2000. Results include age-adjusted rates for all, estrogen receptor (ER)–positive and ER-negative breast cancer patients. ER-specific rates were calculated as the number of cases of that subtype divided by all women; in this manner, rates of both subtypes sum to the overall breast cancer rates. Actual US rates are derived from Surveillance, Epidemiology, and End Results (SEER) incidence–based mortality data. Unknown cases imputed assuming an 80/20 split between ER+ and ER- prevalence. SEER results by ER status are only shown from 1994 through 2000, before which ER status was not collected or largely unknown. ER = estrogen receptor.
Table 2.
Age-adjusted reduction in breast cancer mortality rates (per 100000 women) in 2000 attributed to adjuvant treatment and/or screening relative to no screening and no adjuvant treatment for women aged 30 to 79 years*
| Model | All breast cancer cases | ER+ breast cancer cases | ER- breast cancer cases | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tam | Chemo | TX | SCR | SCR+TX | Tam | Chemo | TX | SCR | SCR+TX | Tam | Chemo | TX | SCR | SCR+ TX |
|
| D | 6 | 5 | 11 | 9 | 19 | 6 | 2 | 8 | 7 | 15 | 0 | 3 | 3 | 2 | 5 |
| E | 9 | 8 | 16 | 9 | 22 | 9 | 4 | 12 | 7 | 16 | 0 | 4 | 4 | 3 | 6 |
| G-E | 8 | 5 | 13 | 14 | 24 | 8 | 3 | 10 | 10 | 18 | 0 | 3 | 3 | 4 | 6 |
| M | 8 | 4 | 12 | 8 | 15 | 8 | 2 | 10 | 6 | 13 | 0 | 2 | 2 | 2 | 3 |
| S | 13 | 6 | 16 | 14 | 26 | 13 | 3 | 15 | 11 | 21 | 0 | 2 | 2 | 3 | 5 |
| W | 10 | 6 | 16 | 10 | 24 | 10 | 3 | 12 | 7 | 18 | 0 | 4 | 4 | 3 | 6 |
| Median | 8 | 6 | 14 | 10 | 23 | 8 | 3 | 11 | 7 | 17 | 0 | 3 | 3 | 3 | 5 |
* Mortality reductions are absolute reductions, computed as the difference between the rate predicted in the absence of both screening and adjuvant treatment and the rate predicted with one or both interventions. Chemo = chemotherapy; ER = estrogen receptor; SCR = screening mammography; SCR+TX = screening mammography and tamoxifen and chemotherapy; Tam = tamoxifen; TX = tamoxifen and chemotherapy.
Treatment had a greater overall impact on death rates from ER-positive vs ER-negative disease, largely because of tamoxifen use (Table 2, Figure 2). In contrast, screening alone accounted for similar percent reductions in mortality for ER-positive and ER-negative cases (median = 16.7% vs 14.0%, respectively) (Table 3). A comparison of the stage distribution for a representative SEER model (Model S) versus the Breast Cancer Surveillance Consortium (BCSC), among cases stratified by mode of detection, shows reasonable agreement (Table 4); the probability of screen detection by ER are similar relatively, but differ in absolute value because the SEER-modeled and BCSC populations are not the same in terms of the overall proportion screened (Table 4). Even though ER-negative cases were less likely to be screen-detected than ER-positive cases (35.1% vs 51.2%, respectively) (Table 5), when screen-detected, survival benefit was found to be greater for ER-negative cases. To explain this observation, we used Model S to estimate the probability of a stage-shift among screen-detected cases by ER status (Table 5). After removing overdiagnosed cases, we found that screen-detected ER-negative cases were as likely to be downstaged by screening as ER-positive cases (39.1% for ER-negative vs 35.5% for ER-positive cases) (Table 5). Most of the stage shift occurred from regional to local for both subtypes (Table 5), however, because the survival gain of this stage-shift was greater for ER-negative vs -positive cases (25.6% vs 20.2%) (Table 5), the overall five-year survival gain was higher for ER-negative vs -positive cases (35.6% vs 30.7%) (Table 5). Of note, these survival gains assumed no adjuvant treatment. In addition, we found that the age distribution among the screen-detected, stage-shifted patients was similar by ER status, with older women more likely to experience a stage shift than younger women across both ER subtypes: 47.3%–48.6% among women aged 60 to 69 years vs 15.5%–17.6% among women aged 40 to 49 years (Table 5).
Figure 2.
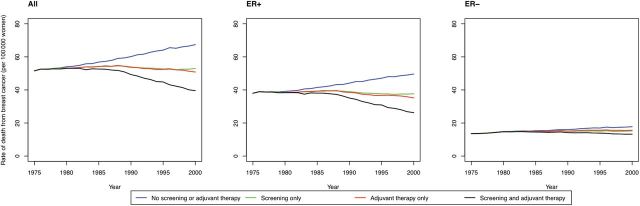
Predicted US overall and estrogen receptor (ER)–specific breast cancer mortality rates under counterfactual scenarios that include no screening and no adjuvant therapy, screening only, adjuvant therapy only, in comparison to screening and adjuvant treatment, for representative model (Model S). Results include age-adjusted rates for all, ER-positive and ER-negative breast cancer patients. ER-specific rates were calculated as the number of cases of that subtype divided by all women; in this manner, rates of both subtypes sum to the overall breast cancer rates. ER = estrogen receptor.
Table 3.
Estimated percent reduction (%) for overall and estrogen receptor–specific breast cancer mortality rates in 2000 attributable to adjuvant treatment and/or screening, relative to no screening and no adjuvant treatment, for women aged 30 to 79 years*
| Model | All breast cancer cases | ER+ breast cancer cases | ER- breast cancer cases | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tam | Chemo | TX | SCR | SCR+TX | Tam | Chemo | TX | SCR | SCR+TX | Tam | Chemo | TX | SCR | SCR+TX | |
| D | 9.7 | 8.0 | 17.3 | 14.8 | 30.2 | 13.8 | 5.5 | 18.8 | 16.0 | 32.7 | 0 | 13.8 | 13.8 | 11.6 | 23.6 |
| E | 13.6 | 12.1 | 24.7 | 14.5 | 35.0 | 19.7 | 9.0 | 27.2 | 15.2 | 37.5 | 0 | 19.1 | 19.1 | 13.0 | 29.2 |
| G-E | 11.1 | 7.9 | 18.5 | 20.0 | 34.6 | 16.1 | 5.7 | 20.5 | 20.8 | 37.0 | 0 | 13.9 | 13.9 | 18.1 | 29.1 |
| M | 14.3 | 7.6 | 22.2 | 15.3 | 28.2 | 19.1 | 4.7 | 24.2 | 15.8 | 31.5 | 0 | 16.4 | 16.4 | 14.0 | 18.6 |
| S | 18.3 | 8.9 | 25.3 | 21.4 | 38.8 | 24.7 | 7.5 | 30.1 | 22.5 | 43.4 | 0 | 13.1 | 13.1 | 14.1 | 25.3 |
| W | 16.4 | 10.5 | 26.3 | 16.4 | 39.4 | 23.4 | 6.8 | 29.4 | 17.4 | 42.9 | 0 | 19.1 | 19.1 | 14.2 | 31.2 |
| Median | 13.9 | 8.5 | 23.4 | 15.9 | 34.8 | 19.4 | 6.3 | 25.7 | 16.7 | 37.3 | 0 | 15.1 | 15.1 | 14.0 | 27.2 |
* Percentages are computed as the difference between the rate predicted in the absence of screening and treatment and the rate predicted with one or both interventions, divided by the rate predicted in the absence of screening and treatment. Chemo = chemotherapy; ER = estrogen receptor; SCR = screening mammography; SCR+TX = screening mammography and tamoxifen and chemotherapy; Tam = tamoxifen; TX = tamoxifen and chemotherapy.
Table 5.
Effect of screening on stage-shift and five-year breast cancer survival by estrogen receptor status in the presence of screening and no adjuvant treatment, for Model S
| Outcome | ER+ cases | ER- cases |
|---|---|---|
| P (screen-detected)* | 51.2% | 35.1% |
| P (stage-shifted | screen-detected)†# | 35.5% | 39.1% |
| P (age | screen-detected, stage-shifted) ‡# | ||
| 40–49 y | 15.5% | 17.6% |
| 50–59 y | 35.8% | 35.1% |
| 60–69 y | 48.6% | 47.3% |
| P (stage shift type | screen-detected, stage-shifted)§# | ||
| Regional → local | 77.5% | 71.6% |
| Distant →local | 13.9% | 15.4% |
| Distant →regional | 8.6% | 13.1% |
| Difference in five-year breast cancer survival probability by stage shift type||# | ||
| Regional →local | 20.2% | 25.6% |
| Distant →local | 74.7% | 72.3% |
| Distant →regional | 54.5% | 46.6% |
| Overall difference in five-year breast cancer survival probability for a screen-detected patient¶# | 30.7% | 35.6% |
* P (screen-detected) denotes the probability of being screen-detected. ER = estrogen receptor.
† P (stage-shifted | screen-detected) denotes the probability of being stage-shifted conditioned on being screen-detected. Note that stage-shifting occurs when patients are screen-detected at an earlier stage than they would have been detected in the absence of screening.
‡ P (age | screen-detected, stage-shifted) denotes the probability of being a specific age conditioned on being screen-detected and being stage-shifted.
§ P (stage shift type | screen-detected, stage-shifted) denotes the probability of experiencing a specific type of stage shift conditioned on being screen-detected and being stage-shifted. Stage shift types are: regional stage in absence of screening and local stage in presence of screening (denoted by “Regional -> local”), distant stage in absence screening and local stage in presence of screening (denoted by “Distant -> local”), and distant stage in absence of screening and regional stage in presence of screening (denoted by “Distant -> regional”).
|| The differences in five-year breast cancer survival probability by stage shift type are computed by comparing with stage-specific survival curves in the absence of screening and treatment by ER status (Supplementary Table 7, available online). For instance, the five-year survival benefit of the regional-to-local stage shift is calculated as the difference observed between the five-year survival probability of the local stage disease and the five-year survival probability of regional stage disease.
¶ The difference in overall five-year breast cancer survival probability is computed by first evaluating the differences in the five-year breast cancer survival probability by stage shift type, then taking a weighted sum of these differences using the distribution of stage shift types as the weights. These results do not account for “within-stage” shift differences, although within-stage shifts were more favorable for ER-negative cases (data not shown).
# Outcome does not include overdiagnosed patients, and are screen-detected patients and die of non–breast cancer causes between the time they were screen-detected and the time they would have been symptomatically detected (ie, the lead time).
Sensitivity Analysis
Compared with mortality reductions seen under actual screening dissemination patterns, the models predicted that additional mortality reductions could have been achieved with annual and biennial screening for all cancers and for cancers by ER status, assuming actual treatment dissemination patterns. Overall, biennial screening captured 85%-94% (range across models) of the mortality reduction obtained with annual screening (Table 6). However, biennial screening captured a lower proportion of the mortality reduction achievable with annual screening for ER-negative vs ER-positive cases (model range = 80%-87% vs 87%-96.5%, respectively). This translated into a slightly greater additional percent mortality reduction under annual screening for ER-negative vs -positive cases (model range = 5%–9% vs 2%–7%, respectively) (Table 6).
Table 6.
Sensitivity analysis on percent reductions for overall and estrogen receptor–specific breast cancer mortality rates in 2000 attributable to adjuvant treatment and/or screening, relative to no screening and no adjuvant treatment, for women aged 30 to 79 years, for Models S and E
| Scenario | Percent reduction (%) in mortality rates in 2000* | |||||
|---|---|---|---|---|---|---|
| All breast cancers cases | ER+ cases | ER- cases | ||||
| SCR | SCR+TX | SCR | SCR+TX | SCR | SCR+TX | |
| Impact of increasing screening frequency, by model | ||||||
| Model S | ||||||
| Base-case (actual) dissemination†‡ | 21.4 | 38.8 | 22.5 | 43.4 | 14.1 | 25.3 |
| Biennial screening and 100% compliance‡ | 34.8 | 49.8 | 37.3 | 54.5 | 27.4 | 36.1 |
| Annual screening and 100% compliance‡ | 39.0 | 52.5 | 41.1 | 56.6 | 33.4 | 41.2 |
| Model E | ||||||
| Base-case (actual) dissemination† | 14.5 | 35.0 | 15.2 | 37.5 | 13.0 | 29.2 |
| Biennial screening and 100% compliance‡ | 24.1 | 43.6 | 25.2 | 46.5 | 21.6 | 37.1 |
| Annual screening and 100% compliance‡ | 35.6 | 51.8 | 37.2 | 54.2 | 31.1 | 46.3 |
| Impact of increasing screening frequency when ER- equals ER+ mammography threshold, by model | ||||||
| Model S | ||||||
| Base-case (actual) dissemination† | 21.9 | 39.1 | 22.5 | 43.4 | 15.7 | 26.5 |
| Biennial screening and 100% compliance | 35.4 | 50.4 | 37.3 | 54.5 | 29.8 | 38.3 |
| Annual screening and 100% compliance | 39.6 | 53.0 | 41.1 | 56.6 | 35.3 | 42.9 |
| Model E | ||||||
| Base-case (actual) dissemination† | 15.2 | 35.5 | 15.2 | 37.5 | 15.2 | 30.7 |
| Biennial screening and 100% compliance | 25.0 | 44.3 | 25.2 | 46.5 | 24.4 | 39.4 |
| Annual screening and 100% compliance | 36.6 | 52.6 | 37.2 | 54.2 | 35.4 | 49.2 |
* Percentages are computed as the difference between the rate predicted in the absence of screening and treatment and the rate predicted with screening or both interventions, divided by the rate predicted in the absence of screening and treatment. In the main text, we refer to the proportion of mortality reduction achievable by biannual screening compared with annual screening; this value is computed as the difference in percent reduction between annual and biennial screening divided by the percent reduction achieved through annual screening. ER = estrogen receptor; SCR = screening mammography; SCR+TX = screening mammography and tamoxifen and chemotherapy.
† Base case scenario assumes overall population-based estimates for actual screening and treatment dissemination patterns.
‡ Mammography threshold variess by ER status, per Methods.
In the base case, we used ER-specific mammography detection thresholds (or sensitivity) (Supplementary Table 1, available online). If we assume that the mammography threshold detection of ER-negative disease was the same as that for ER-positive disease, Models E and S estimated a small additional gain in breast cancer mortality reduction among ER-negative cases (range = 1%–4%) (Table 6), relative to outcomes with ER-specific mammography detection thresholds.
Discussion
This study used six established models to understand how the dissemination of screening and treatment from 1975 to 2000 has impacted breast cancer mortality trends stratified by estrogen receptor status. All six modeling groups concluded that there were greater absolute mortality declines in ER-positive than among ER-negative cancers. The relative contribution of adjuvant treatment vs screening to breast cancer mortality reductions was higher for ER-positive cases; for ER-negative cases, the relative contributions were similar. It is not surprising that there were greater mortality reductions in ER-positive vs negative cases, given the therapies available from 1975–2000. While standard chemotherapy has a larger benefit for ER-negative than ER-positive cases (10,11) only ER-positive cases benefit from tamoxifen. More interestingly, even though ER-negative cases were less likely to be screen-detected, when screen-detected, their survival benefit from screening was somewhat larger because of greater survival gains associated with downstaging. In addition, older women were more likely to experience these stage shifts than younger women because of higher incidence. Finally, increasing screening use could decrease mortality for both molecular subtypes, but biennial screening captures a lower proportion of the mortality reduction than is achievable with annual screening for ER-negative vs ER-positive cases.
The finding that ER-negative cases are less likely to be screen-detected than ER-positives likely reflects natural history differences in duration of the screen-detectable period, with ER-negative cases being more aggressive. This leads to low length-biased sampling (19,33,34) among ER-negative cases, with tumors that are faster growing having poor prognosis and less time to be screen-detected. However, the finding that when ER-negative cancers are screen-detected, they can be downstaged with improved survival suggests that novel screening approaches (35,36) that can detect fast-growing tumors in early stages could further reduce mortality in ER-negative subtypes.
In prior work, we found that biennial screening achieved most of the overall mortality reductions from annual screening (16). Stratified by ER subtype, we find that biennial screening captured a lower proportion of the mortality reduction achievable with annual screening for ER-negative than for ER-positive cases because of faster sojourn time for ER-negative than ER-positive cases. The differential impact of screening interval by ER status implies that women predisposed to develop ER-negative cancer might benefit from annual screening, whereas those who will develop ER-positive disease could be screened less often. BRCA1 mutation carriers, for instance, are an illustrative example of patients who are more likely to develop ER-negative cancers that benefit from intensive screening (37,38).
While screening dissemination was high in the latter part of our observation period, the model results demonstrate that increased compliance with regular screening could have achieved greater mortality reductions by the year 2000 than were actually realized for both molecular sub-types. However, this result does not advocate for more intensive screening in the average risk population. Currently, there is not sufficient data among average individuals on their risk of developing cancer by ER subtype or other molecular subtypes to support a difference in screening regimens by risk of molecular subtype of breast cancer. Moreover, our analysis did not consider the harms of screening (eg, morbidity from surgery for screen-detected disease, decrements in quality of life associated with false-positive results, or overdiagnosis) (16,39–41).
Despite the consistent results across the models, our analysis has several limitations. First, the impact of systemic therapy by molecular subtype has shifted over time with the more recent molecular subcategorization of breast cancers by human epidermal growth factor receptor 2 (HER2) status and the evolution of newer chemotherapy regimens (eg, trastuzumab and taxanes) (42–45), yet we did not model HER2 status or use of trastuzumab and other modern therapeutics because these tests and therapies were not in practice until after 2000 (46).
Additionally, since SEER only began collecting HER2 data in 2009 and HER2-specific clinical trials do not have many years of follow-up, there are limited long-term outcomes data to inform modeling by HER2. For ER-negative, HER2-positive cases, it is possible that the potential reductions in mortality with annual screening reported here may be offset by the efficacy of therapies such as trastuzumab. However, for ER-negative disease that is HER2- and PR-negative (“triple negative”), because only chemotherapy is used, our results may be more informative.
Another limitation is that we only considered plain film mammography because it was the primary modality used from 1975 to 2000. There is no evidence as yet suggesting that digital screening will perform better in detecting ER-negative cancers than plain film (19), but other modalities such as MRI, PET scans, and tomosynthesis may have superior detection of the faster-growing molecular subtypes. We found that varying assumptions about screening detection thresholds by ER status had only a small impact on mortality. This result suggests that any new screening technology will need to have a moderate increase in detection to impact population-level mortality. Finally, while all six models produced qualitatively similar results and reproduced our earlier work for all cases combined (12), there were some quantitative differences in the magnitude of screening and/or treatment impacts by ER status across models based on differences in structure and assumptions.
Even with these acknowledged limitations, this research represents the first population-level analysis quantifying the contributions of screening mammography and treatment with tamoxifen and chemotherapy by ER subtype. The conclusions from six collaborating modeling groups about the impact of screening and treatment on mortality among ER-negative and -positive breast cancers were robust and provide greater credibility than inferences based on one model alone. Overall, our results suggest that while ER-negative cases are less likely to be screen-detected, when they are screen-detected, they show greater survival gain attributed to screening compared with ER-positive cases. However, at present, we do not have a predictive model to identify average risk women who are most likely to develop ER-negative breast cancer to support alternative screening by ER subtype (47,48). In the future, as molecular markers better characterize breast cancer subtypes and risk factors of specific subtypes are identified, comparative model-based analysis can be useful to address complex public health questions related to the use of molecularly targeted screening regimens at the population level.
Funding
This research was supported by grant number U01 CA152958 from the National Cancer Institute as part of the Cancer Intervention and Surveillance Modeling Network. Data were provided by the National Cancer Institute-funded Breast Cancer Surveillance Consortium (HHSN261 201100031C).
Supplementary Material
The funders had no role in design of the study, the collection, analysis, or interpretation of the data, the writing of the manuscript, nor the decision to submit the manuscript for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
We thank Drs. Allison Kurian and Chin Hur for valuable comments and suggestions on the manuscript. We also thank the Breast Cancer Surveillance Consortium (BCSC) investigators, participating women, mammography facilities, and radiologists for the deidentified data they have provided for this study. Data were provided by the National Cancer Institute–funded BCSC (HHSN261 201100031C). A list of the BCSC investigators and procedures for requesting BCSC data for research purposes are provided at: http://breastscreening.cancer.gov. The collection of cancer and vital status data used in this study was supported in part by several state public health departments and cancer registries throughout the United States. For a full description of these sources, please see: http://breastscreening.cancer.gov/work/acknowledgement.html.
References
- 1. Kurian AW, Carlson RW. Chapter 17: Principles of Breast Cancer Therapy. In: Li CI, (ed). Breast Cancer Epidemiology: Springer; 2010:371–388. [Google Scholar]
- 2. Sedjo RL, Byers T, Barrera E, Jr, et al. A midpoint assessment of the American Cancer Society challenge goal to decrease cancer incidence by 25% between 1992 and 2015. CA Cancer J Clin. 2007;57(6):326–340. [DOI] [PubMed] [Google Scholar]
- 3. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. [DOI] [PubMed] [Google Scholar]
- 4. Esserman L, Shieh Y, Thompson I. Rethinking screening for breast cancer and prostate cancer. JAMA. 2009;302(15):1685–1692. [DOI] [PubMed] [Google Scholar]
- 5. Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59(4):225–249. [DOI] [PubMed] [Google Scholar]
- 6. Ludwig JA, Weinstein JN. Biomarkers in cancer staging, prognosis and treatment selection. Nat Rev Cancer. 2005;5(11):845–856. [DOI] [PubMed] [Google Scholar]
- 7. Oakman C, Bessi S, Zafarana E, et al. Recent advances in systemic therapy: new diagnostics and biological predictors of outcome in early breast cancer. Breast Cancer Res. 2009;11(2):205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57(2):75–89. [DOI] [PubMed] [Google Scholar]
- 9. Sotiriou C, Pusztai L. Gene-expression signatures in breast cancer. N Engl J Med. 2009;360(8):790–800. [DOI] [PubMed] [Google Scholar]
- 10. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687–1717. [DOI] [PubMed] [Google Scholar]
- 11. Berry DA, Cirrincione C, Henderson IC, et al. Estrogen-receptor status and outcomes of modern chemotherapy for patients with node-positive breast cancer. JAMA. 2006;295(14):1658–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Berry DA, Cronin KA, Plevritis SK, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353(17):1784–1792. [DOI] [PubMed] [Google Scholar]
- 13. Bleyer A, Welch HG. Effect of three decades of screening mammography on breast-cancer incidence. N Engl J Med. 2012;367(21):1998–2005. [DOI] [PubMed] [Google Scholar]
- 14. Kerlikowske K, Zhu W, Hubbard RA, et al. Outcomes of Screening Mammography by Frequency, Breast Density, and Postmenopausal Hormone Therapy. JAMA Intern Med. 2013;173(9):807–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jatoi I, Anderson WF, Jeong JH, et al. Breast cancer adjuvant therapy: time to consider its time-dependent effects. J Clin Oncol. 2011;29(17):2301–2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mandelblatt JS, Cronin KA, Bailey S, et al. Effects of mammography screening under different screening schedules: model estimates of potential benefits and harms. Ann Intern Med. 2009;151(10):738–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nystrom L, Andersson I, Bjurstam N, et al. Long-term effects of mammography screening: updated overview of the Swedish randomised trials. Lancet. 2002;359(9310):909–919. [DOI] [PubMed] [Google Scholar]
- 18. Olsen O, Middleton P, Ezzo J, et al. Quality of Cochrane reviews: assessment of sample from 1998. BMJ. 2001;323(7317):829–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kerlikowske K, Hubbard RA, Miglioretti DL, et al. Comparative effectiveness of digital versus film-screen mammography in community practice in the United States: a cohort study. Ann Intern Med. 2011;155(8):493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Berry DA, Inoue L, Shen Y, et al. Modeling the impact of treatment and screening on U.S. breast cancer mortality: a Bayesian approach. J Natl Cancer Inst Monogr. 2006;(36):30–6. [DOI] [PubMed] [Google Scholar]
- 21. Clarke LD, Plevritis SK, Boer R, et al. A comparative review of CISNET breast models used to analyze U.S. breast cancer incidence and mortality trends. J Natl Cancer Inst Monogr. 2006;(36):96–105. [DOI] [PubMed] [Google Scholar]
- 22. Fryback DG, Stout NK, Rosenberg MA, et al. The Wisconsin Breast Cancer Epidemiology Simulation Model. J Natl Cancer Inst Monogr. 2006;(36):37–47. [DOI] [PubMed] [Google Scholar]
- 23. Mandelblatt J, Schechter CB, Lawrence W, et al. The SPECTRUM population model of the impact of screening and treatment on U.S. breast cancer trends from 1975 to 2000: principles and practice of the model methods. J Natl Cancer Inst Monogr. 2006;(36):47–55. [DOI] [PubMed] [Google Scholar]
- 24. Plevritis SK, Sigal BM, Salzman P, et al. A stochastic simulation model of U.S. breast cancer mortality trends from 1975 to 2000. J Natl Cancer Inst Monogr. 2006;(36):86–95. [DOI] [PubMed] [Google Scholar]
- 25. Tan SY, van Oortmarssen GJ, de Koning HJ, et al. The MISCAN-Fadia continuous tumor growth model for breast cancer. J Natl Cancer Inst Monogr. 2006;(36):56–65. [DOI] [PubMed] [Google Scholar]
- 26. Holford TR, Cronin KA, Mariotto AB, et al. Changing patterns in breast cancer incidence trends. J Natl Cancer Inst Monogr. 2006;(36):19–25. [DOI] [PubMed] [Google Scholar]
- 27. Human Mortality Database. Berkeley: University of California, Berkeley. Available at: http://www.mortality.org Accessed September 30, 2005.
- 28. Cronin KA, Yu B, Krapcho M, et al. Modeling the dissemination of mammography in the United States. Cancer Causes Control. 2005;16(6):701–712. [DOI] [PubMed] [Google Scholar]
- 29. Mariotto AB, Feuer EJ, Harlan LC, et al. Dissemination of adjuvant multiagent chemotherapy and tamoxifen for breast cancer in the United States using estrogen receptor information: 1975–1999. J Natl Cancer Inst Monogr. 2006;(36):7–15. [DOI] [PubMed] [Google Scholar]
- 30. Cronin KA, Mariotto AB, Clarke LD, et al. Additional common inputs for analyzing impact of adjuvant therapy and mammography on U.S. mortality. J Natl Cancer Inst Monogr. 2006;(36):26–29. [DOI] [PubMed] [Google Scholar]
- 31. Mariotto A, Feuer EJ, Harlan LC, et al. Trends in use of adjuvant multi-agent chemotherapy and tamoxifen for breast cancer in the United States: 1975–1999. J Natl Cancer Inst. 2002;94(21):1626–1634. [DOI] [PubMed] [Google Scholar]
- 32. Polychemotherapy for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet. 1998;352(9132):930–942. [PubMed] [Google Scholar]
- 33. Fowble BL, Schultz DJ, Overmoyer B, et al. The influence of young age on outcome in early stage breast cancer. Int J Radiat Oncol Biol Phys. 1994;30(1):23–33. [DOI] [PubMed] [Google Scholar]
- 34. Jayasinghe UW, Taylor R, Boyages J. Is age at diagnosis an independent prognostic factor for survival following breast cancer? ANZ J Surg. 2005;75(9):762–767. [DOI] [PubMed] [Google Scholar]
- 35. Fehm T, Hoffmann O, Aktas B, et al. Detection and characterization of circulating tumor cells in blood of primary breast cancer patients by RT-PCR and comparison to status of bone marrow disseminated cells. Breast Cancer Res. 2009;11(4):R59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lianidou ES, Markou A. Circulating tumor cells in breast cancer: detection systems, molecular characterization, and future challenges. Clin Chem. 2011;57(9):1242–1255. [DOI] [PubMed] [Google Scholar]
- 37. Kurian AW, Munoz DF, Rust P, et al. Online Tool to Guide Decisions for BRCA1/2 Mutation Carriers. J Clin Oncol. 2012;30(5):497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kurian AW, Sigal BM, Plevritis SK. Survival analysis of cancer risk reduction strategies for BRCA1/2 mutation carriers. J Clin Oncol. 2010;28(2):222–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bonomi AE, Boudreau DM, Fishman PA, et al. Quality of life valuations of mammography screening. Qual Life Res. 2008;17(5):801–814. [DOI] [PubMed] [Google Scholar]
- 40. El-Tamer MB, Ward BM, Schifftner T, et al. Morbidity and mortality following breast cancer surgery in women: national benchmarks for standards of care. Ann Surg. 2007;245(5):665–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. van Ravesteyn NT, Miglioretti DL, Stout NK, et al. Tipping the balance of benefits and harms to favor screening mammography starting at age 40 years: a comparative modeling study of risk. Ann Intern Med. 2012;156(9):609–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Goldhirsch A, Gelber RD, Piccart-Gebhart MJ, et al. Two years versus 1 year of adjuvant trastuzumab for HER2-positive breast cancer (HERA): an open-label, randomised controlled trial. Lancet. 2013;382(9897):1021–1028. [DOI] [PubMed] [Google Scholar]
- 43. Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353(16):1659–1672. [DOI] [PubMed] [Google Scholar]
- 44. Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353(16):1673–1684. [DOI] [PubMed] [Google Scholar]
- 45. Slamon D, Eiermann W, Robert N, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365(14):1273–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Amer Soc Clinical Oncology. Available at: http://www.asco.org/guidelines/breast-cancer. Accessed October 08, 2013.
- 47. Chlebowski RT, Anderson GL. The influence of time from menopause and mammography on hormone therapy-related breast cancer risk assessment. J Natl Cancer Inst. 2011;103(4):284–285. [DOI] [PubMed] [Google Scholar]
- 48. Farhat GN, Parimi N, Chlebowski RT, et al. Sex Hormone Levels and Risk of Breast Cancer With Estrogen Plus Progestin. J Natl Cancer Inst. 2013;105(19):1496–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


