Figure 2.
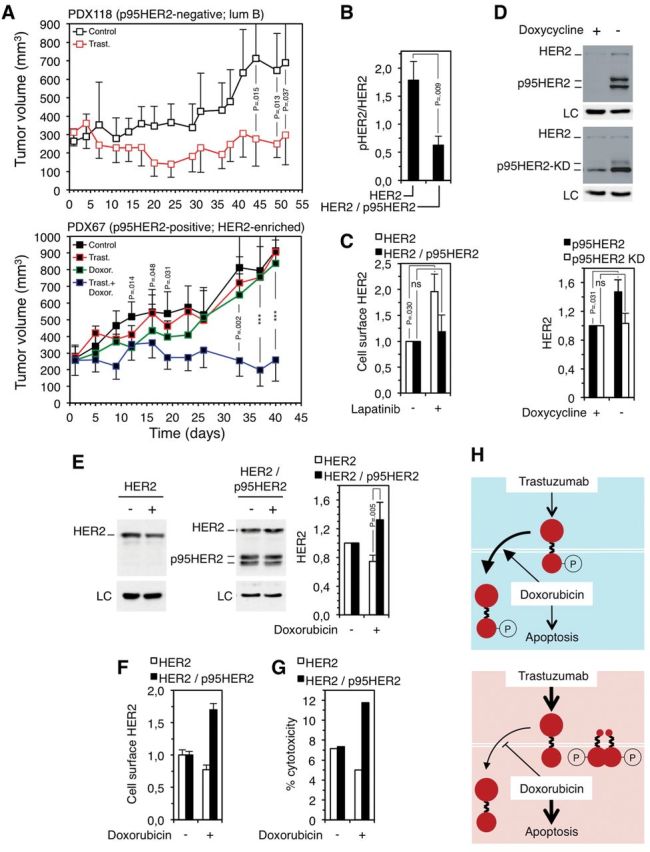
Effect of trastuzumab and doxorubicin on the growth of p95HER2/611CTF-negative and -positive patient-derived xenografts (PDXs) and effect of doxorubicin on human epidermal growth factor receptor 2 (HER2) levels in p95HER2/611CTF-negative and -positive cells. A) Patient-derived xenografts obtained from two HER2-positive breast cancers not expressing (upper panel) (PDX118) or expressing (lower panel) (PDX67) p95HER2/611CTF (11) were treated as indicated (n = 7 in each group). Error bars correspond to 95% confidence intervals. P values were calculated using the two-sided Student’s t test; ***P < .001 (PDX67; control mice vs mice treated with doxorubicin at days 12-19; mice treated with trastuzumab alone vs mice treated with trastuzumab plus doxorubicin at days 33-40). PDX118 and PDX67 belong to the luminal B and HER2-enriched subgroups, according to the gene signatures described in Figure 1B. B) MCF10A cells expressing HER2 or HER2 and p95HER2/611CTF were analyzed by western blot. The signals corresponding to HER2 or phospho-HER2 (pHER2) were quantified. Error bars correspond to 95% confidence intervals of four independent experiments. P values were calculated using the two-sided Student’s t test. C) The same cells as in (B) were treated with 1µM lapatinib for 48 hours. Then, cells were analyzed by flow cytometry with antibodies against the extracellular domain of HER2. The results from three independent experiments are expressed as average fold increase relative to untreated control. Error bars correspond to 95% confidence intervals of four independent experiments. P values were calculated using the two-sided Student’s t test. D) MCF7 Tet-Off cells stably transfected with constructs encoding wild-type p95HER2/611CTF (upper blot) or p95HER2/611CTF bearing a mutation (K767) that inactivates its kinase domain (lower blot), p95HER2/611CTF KD, under the control of a doxycycline regulated promoter, were cultured with or without doxycycline. Then, cells were lysed and cell lysates analyzed by western blot. The signal corresponding to full-length HER2 was quantified from four independent experiments and averages and 95% confidence intervals are shown. P values were calculated using the two-sided Student’s t test. E) The same cells as in (B) were treated with 1.25 nM doxorubicin for one week, lysed and cell lysates analyzed by western blot; the signals corresponding to full-length HER2 were quantified from three independent experiments and expressed as average fold increase relative to untreated control ± SD. P values were calculated using the two-sided Student’s t test. F) The same cells as in (B) were treated with 1.25 nM doxorubicin for one week and analyzed by flow cytometry with antibodies against the extracellular domain of HER2. Error bars correspond to 95% confidence intervals of four independent experiments. G) Antibody-dependent cell-mediated cytotoxicity (ADCC) was analyzed as described in (15), after treating target cells with or without 1.25 nM doxorubicin for one week. H) Schematic drawing showing the effect of the combination of trastuzumab and doxorubicin on p95HER2/611CTF-negative cells (blue background) or p95HER2/611CTF-positive cells (red background). HER2 is represented by two big filled red circles linked by a broken line, the p95HER2/611CTF constitutively active fragment is represented by a small filled red circle linked with a broken line to a big one. Treatment with doxorubicin induces apoptosis more efficiently in p95HER2/611CTF-positive cells and, additionally, destabilizes phospho-HER2 and stabilizes HER2 in p95HER2/611CTF-negative and -positive cells, respectively. LC = loading control; HER2 = human epidermal growth factor receptor 2; ns = statistically non-significant
