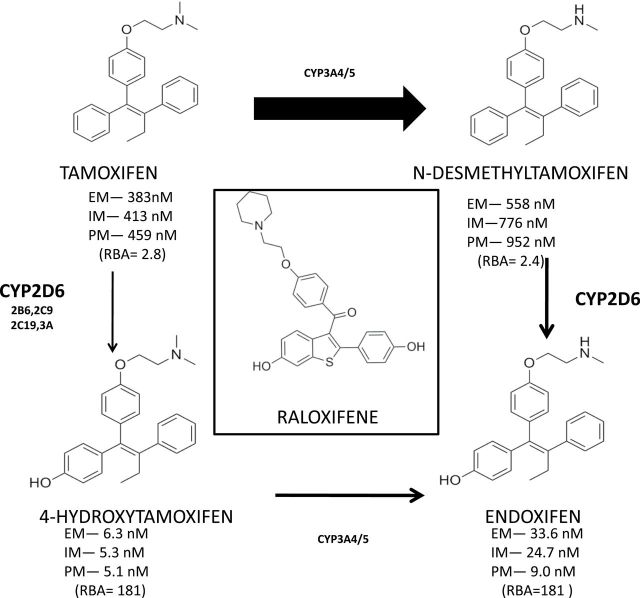
Figure 1.
A diagrammatic representation of relevant tamoxifen metabolism in humans. The thickness of the arrows indicates predominance of pathways with the metabolizing enzymes listed. The relative circulating levels of the parent or the metabolite are reported for extensive metabolizers, intermediate metabolizers, or poor metabolizers by Mürdter et al. (111) based on CYP2D6 genotyping. It can be argued that patients that efficiently metabolize tamoxifen to hydroxylated metabolites (4OHT and endoxifen) with high binding affinity to the estrogen receptor (ER) in an estrogen-rich environment will be better able to block estrogen-stimulated breast cancer cell growth (112). The mix of metabolites will ultimately have an impact on the long-term plasticity of acquired resistant cell populations based on the efficiency of “antiestrogenic pressure” at the tumor ER. Acquired resistance to tamoxifen evolves over five years of retransplantion of ER-positive MCF-7 tumors into athymic mice to become tamoxifen-stimulated but vulnerable to estrogen-induced apoptotic tumor regression (75). The Study of Tamoxifen And Raloxifene showed that during treatment tamoxifen or raloxifene (structure shown center above) produced the same 50% in primary breast cancer (55), but after the five-year treatment was stopped tamoxifen maintained the antitumor action but raloxifene did not and tumor incidence increased (59). Tamoxifen is a long-acting prodrug that maintains high levels of antiestrogen to block the ER; raloxifene is rapidly excreted (57), so lack of compliance will reduce antiestrogenic selection pressure and retard the development of vulnerable populations, sensitive to estrogen-induced apopotosis. The relative binding affinity is based on comparison with E2 as 100. EM = extensive metabolizer; IM = intermediate metabolizer; PM = poor metabolizer; RBA = relative binding affinity.