Abstract
Congenital anomalies of the kidney and urinary tract (CAKUT) affect about 1 in 500 births and are a major cause of morbidity in infants. Duplex collecting systems rank among the most common abnormalities of CAKUT, but the molecular basis for this defect is poorly understood. In mice, conditional deletion of Wnt5a in mesoderm results in bilateral duplex kidney and ureter formation. The ureteric buds (UBs) in mutants emerge as doublets from the intermediate mesoderm (IM)-derived nephric duct (ND) without anterior expansion of the glial cell line-derived neurotrophic factor (Gdnf) expression domain in the surrounding mesenchyme. Wnt5a is normally expressed in a graded manner at the posterior end of the IM, but its expression is down-regulated prior to UB outgrowth at E10.5. Furthermore, ablation of Wnt5a in the mesoderm with an inducible Cre at E7.5 results in duplex UBs, whereas ablation at E8.5 yields normal UB outgrowth, demonstrating that Wnt5a functions in IM development well before the formation of the metanephros. In mutants, the posterior ND is duplicated and surrounding Pax2-positive mesenchymal cells persist in the nephric cord, suggesting that disruption of normal ND patterning prompts the formation of duplex ureters and kidneys. Ror2 homozygous mutants, which infrequently yield duplex collecting systems, show a dramatic increase in incidence with the additional deletion of one copy of Wnt5a, implicating this receptor in non-canonical Wnt5a signaling during IM development. This work provides the first evidence of a role of Wnt5a/Ror2 signaling in IM extension and offers new insights into the etiology of CAKUT and possible involvement of Wnt5a/Ror2 mutations.
INTRODUCTION
Congenital anomalies of the kidney and urinary tract (CAKUT) are among the most common birth defects and a major cause of pediatric morbidity. Duplex kidney formation is included in the spectrum of malformations associated with CAKUT and is characterized by the development of two separate collecting systems with either bifid (merged) or fully duplicated ureters. While duplex kidneys are generally asymptomatic, patients bearing this abnormality may be prone to urinary tract infections (1,2).
The kidney develops from progenitors originating from the intermediate mesoderm (IM). Development is initiated by the invasion of nephric (Wolffian) duct (ND) progenitors along the A–P axis toward the cloaca in the IM and their conversion to an epithelium under the influence of signals provided at least in part from surface ectoderm (3). Subsequently, the metanephric mesenchyme (MM) becomes delineated as a loose tissue mass at the posterior end of the IM adjacent to the ND. Signals emanating from the MM induce the outgrowth of single ureteric buds (UBs) from the ND, which arborizes in the MM beginning at E11.5 in the mouse. The rearranged during transfection (c-RET)/glial cell line-derived neurotrophic factor (GDNF) signaling pathway is the principal regulatory mechanism responsible for bud outgrowth, and its dysregulation results either in no bud outgrowth or supernumerary budding. Thus, several molecules involved in the regulation of c-RET/GDNF expression are critical for normal kidney development (4–6).
In addition to components of the c-RET/GDNF signaling pathway, mutations in molecules associated with the planar cell polarity (PCP) pathway (7,8) also result in duplex kidneys (7,9). However, the mechanism of duplex kidney formation in these mutants has not been clearly investigated. Wnt5a is frequently implicated in non-canonical Wnt/PCP signaling (10). Its loss of function causes several defects in tissue outgrowth from truncation of the A–P axis to shortening of the tail, limbs, digits, face and genitals (11). Also, Wnt5a affects morphogenesis of several organs. Its deletion induces abnormal patterning of the pituitary gland (12), a shortened and bifurcated intestine (13), a shortened and widened cochlea (14), and abnormal outflow tract morphogenesis in the heart (15)—all through non-canonical Wnt/PCP signaling. Ror2 is known to be a receptor for Wnt5a signaling. The phenotypes of Ror2 null mice also show dwarfism, facial abnormalities, short limbs and tails like Wnt5a null mice (16), suggesting that Wnt5a may regulate the morphogenesis of several organs through Ror2.
In this study, we investigated the role of Wnt5a in the development of the metanephros and found that it regulates IM extension, affecting kidney morphogenesis. Wnt5a mutants form a shortened and broadened IM with duplicated NDs at the posterior end prior to UB outgrowth. Thus, the abnormal extension of the posterior ND alters the outgrowth of the UB from the ND, creating a double UB which causes duplex kidney formation. This occurs without a decrease in canonical Wnt activity in the ND. Furthermore, deletion of one Wnt5a allele in Ror2−/− mutants causes haploinsufficiency for the duplex kidney phenotype, implicating Ror2 as the receptor responsible for Wnt5a signaling during IM development. These findings suggest that a defect in the non-canonical Wnt5a/Ror2 signaling pathway may be responsible for duplex kidney formation through dysgenesis of IM extension.
RESULTS
Ablation of Wnt5a using T-Cre results in duplex kidney formation
Since the kidney is derived from the IM, we conditionally inactivated Wnt5a using the T-Cre mouse, which expresses Cre recombinase in nascent primary mesoderm (17). The Wnt5a mutants from this cross showed a shortened A–P body axis, outgrowth defects in the limbs and a tail comparable to that of the Wnt5a null mouse (11). However, unlike the null mutants, the conditional Wnt5a mutant embryos exhibited normal facial structures (Fig. 1A). Since the observed phenotype was limited to mesoderm, it was not overly complicated by abnormalities involving non-mesoderm-derived organ development and the indirect consequences from such defects. The kidneys from mutant embryos exhibited uni- or bilateral renal abnormalities. By H&E staining, mutant kidneys were duplexed and associated with double ureters (Fig. 1B, n = 24/24). Also, whole-mount immunostaining for calbindin clearly showed double ureter formation in the Wnt5a mutant (Fig. 1C).
Figure 1.
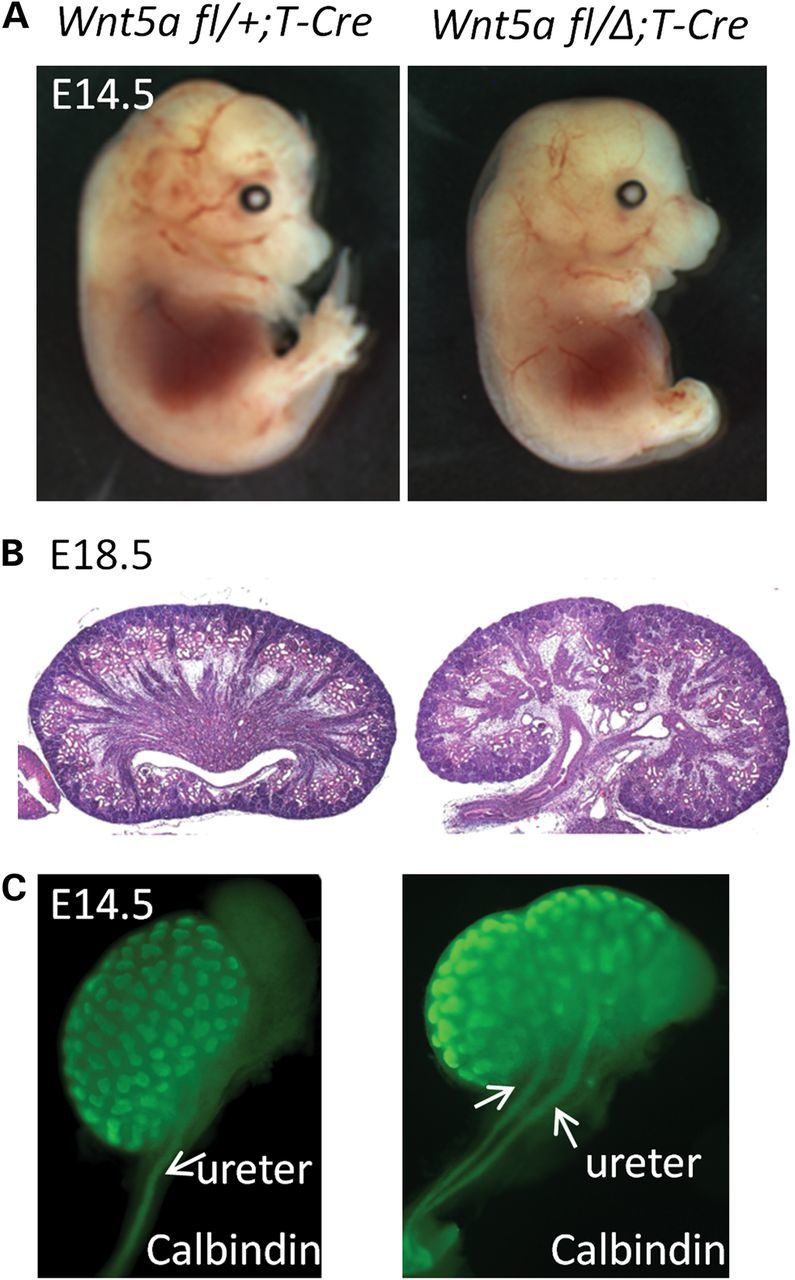
Ablation of Wnt5a using T-Cre results in bilateral duplex kidney formation. (A) left: Wnt5a fl/+;T-Cre embryo at E14.5; right: Wnt5a fl/Δ;T-Cre embryo at E14.5. (B) H&E staining of sagittal sections from Wnt5a fl/+;T-Cre (left) and Wnt5a fl/Δ;T-Cre (right) kidneys at E18.5. (C) Calbindin staining of Wnt5a fl/+;T-Cre and Wnt5a fl/Δ;T-Cre kidneys at E14.5.
The UB in the Wnt5a mutant extends from the ND as a doublet without anterior expansion of the Gdnf expression domain.
To explore the mechanism responsible for double ureter formation, we first examined the outgrowth of the UB. The UB extends from the ND into the adjacent mesenchyme at E10.5, mainly through the interaction of Gdnf, which is secreted by MM cells, with its receptor c-Ret, which is elaborated by the ND (4,6). In other models of duplex kidney development, mutants generally display multiple budding from the ND as a result of dysregulation of c-Ret/Gdnf signaling (1,4,18). We performed whole-mount in situ hybridization (WISH) for c-Ret to evaluate budding at the initiation of kidney development. At E10.5, the ND develops in the mutant as a tubular structure without supernumerary budding, except at the site of normal bud outgrowth (Fig. 2A). Twin buds formed in the mutants, creating double stalks at E11.5 (Fig. 2B). Moreover, these observations were reproducible in explant culture using metanephric rudiments from embryos at E11.5. Wnt5a mutant explants exhibited early signs of duplex kidney formation with double UB outgrowth (Fig. 2C). We also assessed the Gdnf expression domain, as some mutants with a duplex kidney phenotype have an anterior expansion of this domain (1,18). We could not observe an anterior expansion of the Gdnf expression domain in Wnt5a mutants. The dimensions of the domain, however, did reflect the posterior truncation in the embryo, being shorter and wider than the control, but overall, the domain was comparable to that of normal embryos and complemented the pattern observed for c-Ret expression (Fig. 2D). We further evaluated the expression of molecules that regulate UB outgrowth, including Bmp4 (19), Gremlin1 (19) and Sprouty1 (20). Mutants showed a shortened IM and ND, reflecting the truncated A–P body axis, but we found none with expression patterns that were altered from control littermates other than ND marker Sprouty1, which expressed two domains as observed for c-Ret at the posterior end of the ND (Supplementary Material, Fig. S1).
Figure 2.
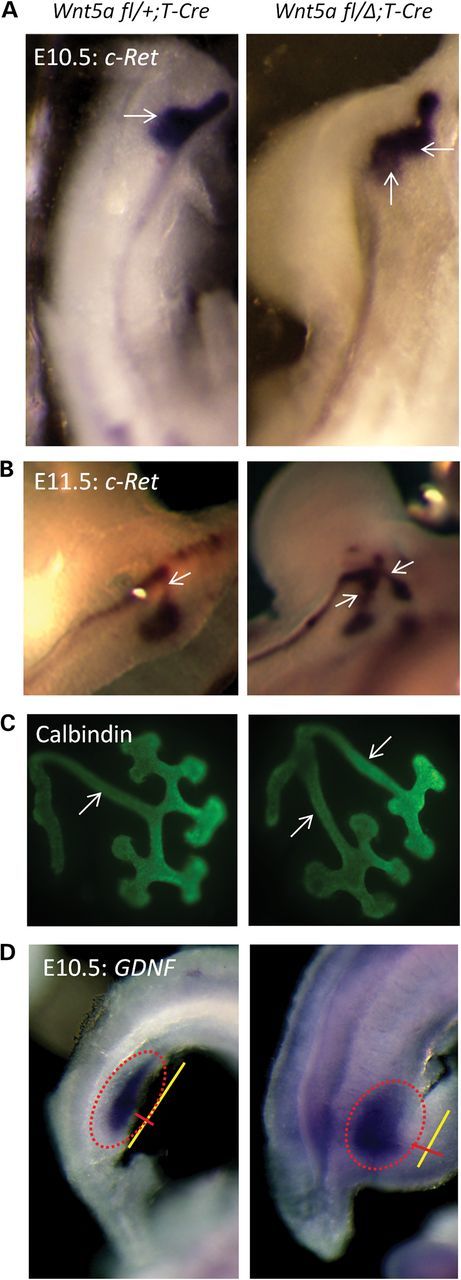
The UB in the Wnt5a mutant grows out as a doublet without anterior expansion of the Gdnf expression domain. (A) WISH for c-Ret using E10.5 embryos. left: Wnt5a fl/+;T-Cre; right: Wnt5a fl/Δ;T-Cre. (B) WISH for c-Ret using E11.5 embryos. left: Wnt5a fl/+;T-Cre; right: Wnt5a fl/Δ;T-Cre. (C) Calbindin staining of E11.5 explants of metanephric rudiments cultured for 24 h. left: Wnt5a fl/+;T-Cre; right: Wnt5a fl/Δ;T-Cre. (D) WISH for Gdnf using E10.5 embryos. left: Wnt5a fl/+;T-Cre; right: Wnt5a fl/Δ;T-Cre. White arrows label the UB (A) or UB stalk (B and C). A red-dotted ellipse marks Gdnf expression (D) with a yellow line for length and red line for width.
Wnt5a ablation during early IM development induces duplex kidney formation
Since the Wnt5a mutant showed outgrowth of the UB as a doublet at the initiation of kidney development, we analyzed the Wnt5a expression pattern in early kidney development. Although Wnt5a is strongly expressed normally in the tail and limb buds, its expression was not demonstrable in the area of UB outgrowth at the time of bud initiation (Supplementary Material, Fig. S2B, yellow ellipse). Even at E11.5, Wnt5a expression was barely detected in the metanephros and then only after lengthy incubations in WISH substrate (Supplementary Material, Fig. S2C, yellow ellipse). This observation is supported by semi-quantitative RT-PCR in which the detection of Wnt5a expression in normal isolated E11.5 MMs or UBs required several more cycles of amplification than for Gdnf or Ret (Supplementary Material, Fig. S2D). These findings suggest that Wnt5a expression at the initiation of metanephric development is not responsible for the suppression of duplex kidney formation.
In addition to the dysregulation of c-Ret/Gdnf expression as a possible cause of duplex kidney formation, some mutants for genes implicated in the PCP signaling pathway, e.g. Ror2 (9) or Fat4 (7), also form duplex kidneys. These mutants, like the Wnt5a mutants, display abnormal A–P body axis extension (7,8) as well. Given that Wnt5a has been demonstrated to function in non-canonical Wnt/PCP signaling (10), defects in this signaling pathway may also be involved in duplex kidney formation. Since the kidney arises from the IM, we hypothesized that the observed defect in Wnt5a mutants may result from the aberrant extension of the IM along the defective A–P axis. Because the ND reaches the cloaca around E10.0, we analyzed embryos for Wnt5a expression during IM elongation at E9.5. Wnt5a was expressed at the posterior end of the IM in a graded manner (Supplementary Material, Fig. S2A)—a pattern comparable to that observed in the tail. This suggests that Wnt5a may function earlier in urogenital development to regulate posterior IM extension and thus cause duplex kidney formation.
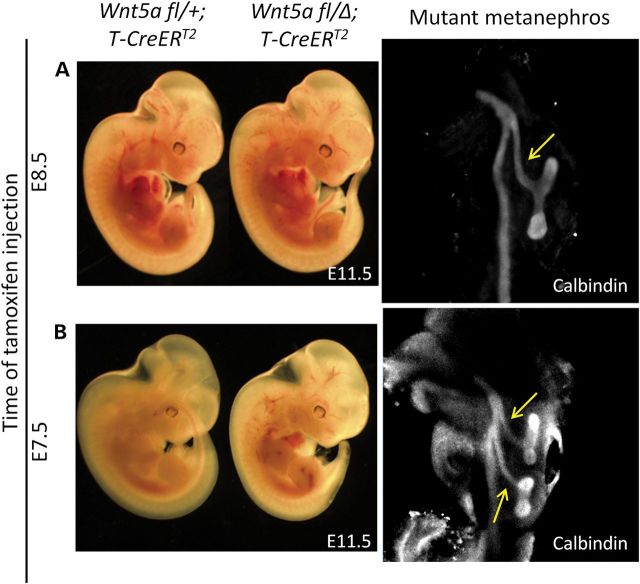
To test whether duplex kidney formation in Wnt5a mutants arises from the abnormal development of the IM, we ablated Wnt5a in a time-dependent and tissue-specific manner using a well-characterized tamoxifen-inducible T-CreERT2 line (21). Tamoxifen was injected at E7.5 or E8.5 and embryos harvested at E11.5 for the formation of double ureters. While tamoxifen injected at E7.5 induced recombination along the entire IM, at E8.5 tamoxifen induced only posterior IM recombination (Supplementary Material, Fig. S3). All treatments activated a Rosa26-YFP reporter allele by E10.5 in the posterior IM from which the UB emanates. Wnt5a ablation during early IM development by tamoxifen injection at E7.5 induced duplex kidney formation bilaterally (n = 5/6) or unilaterally (n = 1/6) (Fig. 3B), whereas Wnt5a ablation by tamoxifen injection at E8.5 failed to elicit the phenotype (Fig. 3A, n = 7/7). This finding suggests that the cause of double UB outgrowth in Wnt5a mutants is associated with early IM morphogenesis.
Figure 3.
Wnt5a ablation in the early IM induces duplex kidney formation. (A) Tamoxifen was injected at E8.5 and embryos were harvested at E11.5. left: Wnt5a fl/+;T-CreERT2; middle: Wnt5a fl/Δ;T-CreERT2; right: metanephros from mutant embryo were stained with calbindin antibody. (B) Tamoxifen was injected at E7.5 and embryos were harvested at E11.5. left: Wnt5a fl/+;T-CreERT2, middle: Wnt5a fl/Δ; T-CreERT2, right: metanephros from mutant embryo were stained with calbindin antibody. The yellow arrows label the UB stalk.
The IM is shortened and broadened, and the ND is duplicated at its caudal terminus in the Wnt5a mutant at E9.5
Since Wnt5a is expressed at the posterior end of the IM at E9.5, we examined its role in ND morphogenesis using the Hoxb7-myr-venus-YFP reporter. The reporter is active in the ND but not the surrounding mesenchyme in the nephric cord as shown by the coincidental expression of E-cadherin that outlines the epithelial cells of the ND (Supplementary Material, Fig. S4). We also examined IM morphogenesis with staining for Pax2, which is expressed in both the ND and nephric cord (Supplementary Material, Fig. S5). The domain of Pax2 expression is significantly shortened and broadened in the mutant (Fig. 4A and Supplementary Material, Fig. S6). The ND itself, as shown with the Hoxb7-myr-venus-YFP reporter, is thickened even in the middle of the mutant IM (Supplementary Material, Fig. S7). Moreover, the posterior end of the duct is especially abnormally thickened, i.e. hypercellular, in the mutant (Fig. 4B and C, and Supplementary Material, Fig. S8). The combination of Hoxb7-myr-venus-YFP reporter activity with Pax2 staining in transverse sections of the posterior end of the E9.5 embryo revealed that, in contrast to the normal ND, the mutant duct is duplicated, showing two foci of Hoxb7-myr-venus-YFP activity (Fig. 4D and E, and Supplementary Material, Fig. S8). The duplication is most often observed as bilateral fusions, such as demonstrated in mutants in Supplementary Material, Fig. S8; however, it may also manifest at the caudal end as distinct ND structures (Fig. 4D). These findings are consistent with our hypothesis that the abnormality precedes the outgrowth of the UB in the Wnt5a mutant.
Figure 4.
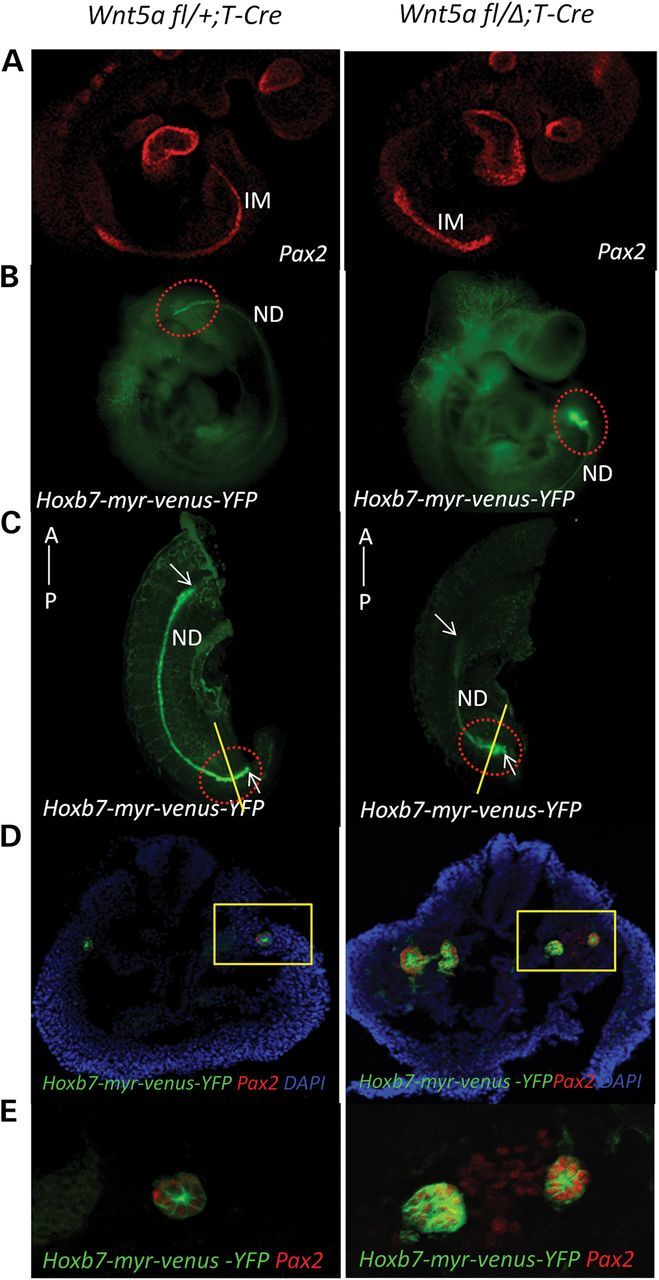
The IM is shortened and broadened in the E9.5 Wnt5a mutant and manifests a duplicated ND at its caudal terminus. (A) Embryos at E9.5 were stained with Pax-2 antibody. left: Wnt5a fl/+;T-Cre; right: Wnt5a fl/Δ;T-Cre. (B) left: Wnt5a fl/+;T-Cre;Hoxb7-myr-venus-YFP embryo at E9.5; right: Wnt5a fl/Δ;T-Cre;Hoxb7-myr-venus-YFP embryo at E9.5. A red-dotted ellipse marks the posterior ends of the embryos. (C) Embryo images without the head using light sheet microscopy. A red-dotted ellipse marks the posterior end of the embryos. White arrows mark the anterior and posterior ends of the ND. (D) Transverse sections of the posterior end of the IM in E9.5 embryos were stained for Pax-2 expression. left: Wnt5a fl/+;T-Cre;Hoxb7-myr-venus-YFP embryo at E9.5; right: Wnt5a fl/Δ;T-Cre;Hoxb7-myr-venus-YFP embryo at E9.5. A yellow rectangle marks the IM. (E) Enlarged image of the IM in (D).
Non-canonical Wnt5a/Ror2 signaling regulates IM extension
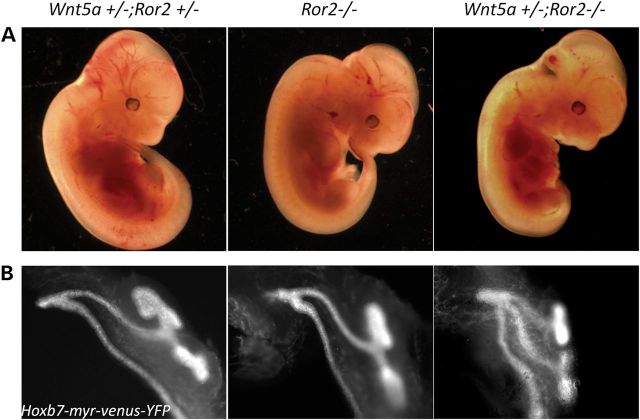
Since Wnt5a can also activate β-catenin-dependent canonical Wnt signaling (22,23), we analyzed mutant and normal embryos for T cell factor (TCF) activation using the BATLacZ mouse (24). β-Catenin activity was detected in both ND structures found in the abnormal posterior of the mutant (Supplementary Material, Fig. S9). However, TCF-dependent activity was not decreased in the Wnt5a mutant (Supplementary Material, Fig. S9), indicating that Wnt5a signaling in the IM is likely through a β-catenin-independent non-canonical Wnt mechanism. The orphan receptors, Ror1 and Ror2, are established Wnt5a receptors and involved in the non-canonical Wnt/PCP signaling pathway (10). The phenotypes of Ror2 null mice are similar to those of Wnt5a null mice showing dwarfism, facial abnormalities, short limbs and tails (16), suggesting that Ror2 is also a receptor for Wnt5a signaling during embryonic development. Moreover, mutations in both Ror2 (25) and Wnt5a (26) have been implicated in the rare genetic disease, Robinow syndrome, which exhibits some similar defects as found in the Wnt5a null mouse such as dwarfism and genital abnormalities (27). Therefore, Ror2 may serve as a receptor for Wnt5a in IM morphogenesis. To test this, we generated mice that were null for Ror2 and heterozygous for Wnt5a. In controls that were heterozygous for both Wnt5a and Ror2, single ureters were always observed; however, one-third of Ror2 null mice already showed a duplex kidney phenotype (Fig. 5 and Table 1). With the additional deletion of a single copy of Wnt5a, the occurrence of the duplex kidney phenotype increased to >90% (Fig. 5B and Table 1). Furthermore, Wnt5a +/−;Ror2 −/− embryos at E9.5 manifested the same shortened IM (Fig. 6A) with abnormal posterior ND as the Wnt5a mutants (Fig. 6B and C). These results are consistent with the established relationship between Wnt5a and Ror2 in the development of other mesodermal tissues, e.g. limb patterning (28).
Figure 5.
Ror2 mediates Wnt5a signaling in duplex kidney formation. Embryos at E11.5 (A) and metanephros from the embryos (B) left: Wnt5a +/−;Ror2 +/−; Hoxb7-myr-venus-YFP; middle: Ror2 −/−;Hoxb7-myr-venus-YFP; right: Wnt5a +/−;Ror2 −/−;Hoxb7-myr-venus-YFP.
Table 1.
Frequency of the duplex kidney phenotype in Ror2 mutants
| Genotype | Wnt5a +/−; Ror2 +/− | Ror2 −/− | Wnt5a +/−; Ror2 −/− |
|---|---|---|---|
| No. of embryos with duplex kidney(s)/no. of total embryos | 0/53 | 8/24 | 24/26 |
| Percentage (%) | 0 | 33.3 | 92.3 |
Figure 6.
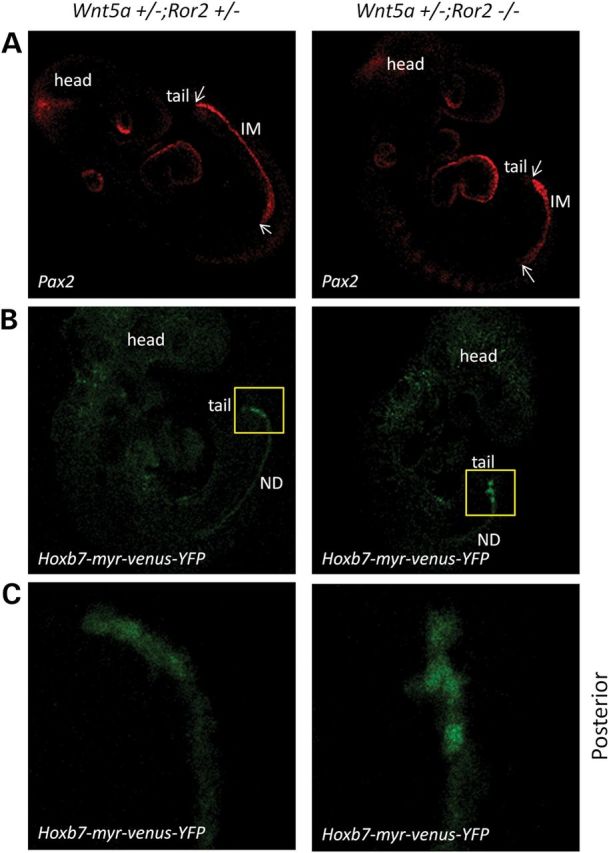
Ror2 mutants also show abnormal IM and ND extension. (A) Embryos at E9.5 were stained with Pax-2 antibody. left: Wnt5a +/−;Ror2 +/−; right: Wnt5a +/−;Ror2 −/−. White arrows mark the anterior and posterior ends of the IM. (B) Embryos at E9.5. left: Wnt5a +/−;Ror2 +/−;Hoxb7-myr-venus-YFP; right: Wnt5a +/−;Ror2 −/−;Hoxb7-myr-venus-YFP. A yellow rectangle marks the posterior ends of the embryos. (C) Enlarged image of posterior ND in (B).
DISCUSSION
Wnt5a controls the morphogenesis of several tissues by providing spatial limitations on cell positioning during tissue elongation. Its loss therefore typically yields shorter and wider structures due, at least in part, to the dysregulation of the non-canonical Wnt/PCP pathway (11,13,14). In the current study, Wnt5a ablation resulted in a shorter and wider ND, consistent with dysfunctional non-canonical Wnt signaling. The bifurcated ND affects subsequent metanephric development presumably through the permissive outgrowth of double UBs, resulting in duplex kidney formation and twin ureters. In other words, Wnt5a regulates not only outgrowth from the primary body axis (11), but also outgrowth of the UB from the ND by controlling IM extension. The exact mechanism of its activity is a major problem in the field. In our particular model, the dysmorphic posterior ND could stem from an aberrant orientation of cells during cell division, abnormal cell migration in the IM or abnormal intercalation of cells in the ND, behaviors consistent with a defective non-canonical Wnt/ PCP pathway (29). These possibilities are currently under investigation.
During preparation of this manuscript, Nishita et al. (30) published an article with the title ‘Role of Wnt5a-Ror2 signaling in morphogenesis of the metanephric mesenchyme during ureteric budding’. Like us, they investigated a similar phenotype, i.e. duplicated ureters in the Wnt5a- and Ror2 null mice. They suggested that dysregulated positioning of the MM causes a spatio-temporally aberrant interaction between the MM and ND, providing the ND with inappropriate Gdnf signaling together with reduced proliferation in mutant MM, and thus resulting in ectopic UB induction. However, we found that early IM extension, especially abnormal duplication of the posterior ND, is the likely cause of duplex kidney formation in the Wnt5a mutants. As mentioned earlier, we also examined Wnt5a expression by RT-PCR of isolated UBs and MMs from the E11.5 metanephros. This was done because Wnt5a WISH data revealed almost no expression of Wnt5a in the metanephric region during initial UB formation, compared with its strong expression in the tail and limbs in a gradient manner. Wnt5a was very weakly expressed based on results from PCR amplification in the E11.5 metanephros in both UB and MM, in agreement with our WISH data. We could detect a Wnt5a PCR product at 43 reaction cycles, while Gapdh was detected at 27 reaction cycles using the same cDNA (Supplementary Material, Fig. S2). To determine if this expression is sufficient to induce double ureter formation, we produced Wnt5a mutants using several Cre lines which ablate Wnt5a in both the MM and UB, e.g Dll1-Cre. However, Wnt5a mutants generated with Dll1-Cre do not show a double ureter phenotype, but their kidneys form normally except that they develop a hydronephrotic kidney phenotype at E16.5 (unpublished data). It was in fact this observation that prompted us to focus on earlier IM extension prior to UB outgrowth. Furthermore, our time-dependent analysis of Wnt5a deletion clearly showed that only early deletion of Wnt5a can cause double ureter formation. Wnt5a mutants with tamoxifen injection at E7.5 formed duplex kidneys. However, Wnt5a mutants with tamoxifen injection even at E8.5 did not yield a duplex kidney phenotype (Fig. 3)—a finding consistent with abnormal IM morphogenesis.
The IM develops along the A–P axis, and Wnt5a is expressed both in the tail bud and in the posterior end of the IM, where we observed that it is distributed in a graded manner. Since the Wnt5a null mutant is truncated along the A–P axis, Wnt5a is regarded as a significant factor in A–P axis extension (11). We observed that the duplex kidney phenotype is always accompanied by truncation of the A–P axis in the Wnt5a mutants, suggesting that IM extension is controlled together with A–P axis extension. It is likely that both are affected by a Wnt5a gradient from early embryonic development, and that an IM extension defect is the result of an accumulation of dysmorphic events that may be maximal at the time ND reaches the cloaca, thus causing the abnormal ND duplication in the Wnt5a mutants. The relationship between the A–P axis and IM extension, however, needs further clarification.
In conclusion, our studies establish a role for Wnt5a in the regulation of IM extension and suggest that duplex kidneys result from the dysmorphogenesis of the posterior ND.
MATERIALS AND METHODS
Animal and tissue culture
Generation, maintenance and genotyping of Wnt5a flox/flox mice (Wnt5atm1.1Tpy) (31), T-Cre (17) mice, Hoxb7/myr-Venus-YFP (32), BATLacZ (24), Ror2 (8) and T-CreERT2 (21) mice have been described previously (33). Noon on the day of vaginal plug detection was designated E0.5. Mice were managed according to the NIH guidelines for the care and use of laboratory animals. Metanephric rudiments were dissected from E11.5 mouse embryos and were cultured on type IV collagen-coated filters, as previously described (17), in DMEM/F12 (50 : 50) with 10% fetal calf serum in 5% CO2 at 37°C. For BATLacZ mice, X-gal staining was performed as previously described (24).
Tamoxifen injections and embryo analyses
About 1.0 mg of tamoxifen (Sigma, T-5648) was administered at either E7.5 or E8.5 by IP injection as previously described (34) without progesterone. The tamoxifen-injected mice were harvested at E11.5 and their kidneys were immunostained for calbindin, which labels the ureter and UB/collecting duct.
In situ hybridization and immunohistochemistry
Embryos were fixed and processed for WISH as described (35). Also, embryos were fixed and processed for paraffin section as described (36). Deparaffinized tissues were probed with Pax-2 antibody (Abcam) at 1 : 50 and visualized using a Dako Cytomation kit according to the manufacturer's instructions.
Immunostaining
Calbindin staining of kidneys or kidney explant cultures was performed according to a protocol for immunostaining described in Kitagaki et al. (33) with some modifications. Briefly, dissected embryonic kidneys were fixed in ice-cold 100% methanol for 10 min followed by two brief washes in phosphate-buffered saline containing 0.1% Triton X-100 (PBST). All subsequent washes and incubations were carried out in PBST. The samples were blocked in 10% heat-inactivated sheep serum (Sigma, St Louis, MO, USA) for 2 h at room temperature, washed and incubated with a 1 : 1000 dilution of calbindin polyclonal antibody (Millipore, Billerica, MA, USA) overnight at 4°C. After three washes, they were incubated with Alexafluor 488 secondary antibodies (Invitrogen) at 1 : 2000 dilution overnight at 4°C. After extensive washes, the embryonic kidneys were photographed by fluorescence microscopy (Zeiss Axio Observer). Likewise, Pax2 staining of embryos or embryo transverse sections was performed with a 1 : 100 dilution of Pax2 polyclonal antibody (Invitrogen) and Alexafluor 568 secondary antibodies (Invitrogen) at 1 : 2000 dilution.
SUPPLEMENTARY MATERIAL
FUNDING
This work was supported by the National Institutes of Health, National Cancer Institute and Center for Cancer Research.
Supplementary Material
ACKNOWLEDGEMENTS
We thank members of the Perantoni lab for their critical review of the manuscript. We also thank Dr Y. Minami (Department of Physiology and Cell Biology, Graduate School of Medicine, Kobe University, Japan) for providing the Ror2 mouse line. We are grateful to C. Hubbard (SAIC) for excellent mouse husbandry. We thank Dr J. Zhu (Cancer and Developmental Biology Laboratory, NCI-Frederick) for help with studies involving tamoxifen-induced Cre activation. We thank OMAL for help with confocal microscope imaging and Zeiss for light sheet microscope imaging.
Conflict of Interest statement. None declared.
REFERENCES
- 1.Kume T., Deng K., Hogan B.L. Murine forkhead/winged helix genes Foxc1 (Mf1) and Foxc2 (Mfh1) are required for the early organogenesis of the kidney and urinary tract. Development. 2000;127:1387–1395. doi: 10.1242/dev.127.7.1387. [DOI] [PubMed] [Google Scholar]
- 2.Whitten S.M., Wilcox D.T. Duplex systems. Prenat. Diagn. 2001;21:952–957. doi: 10.1002/pd.206. [DOI] [PubMed] [Google Scholar]
- 3.Obara-Ishihara T., Kuhlman J., Niswander L., Herzlinger D. The surface ectoderm is essential for nephric duct formation in intermediate mesoderm. Development. 1999;126:1103–1108. doi: 10.1242/dev.126.6.1103. [DOI] [PubMed] [Google Scholar]
- 4.Dressler G.R. Advances in early kidney specification, development and patterning. Development. 2009;136:3863–3874. doi: 10.1242/dev.034876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Costantini F. Genetic controls and cellular behaviors in branching morphogenesis of the renal collecting system. Wiley Interdiscip. Rev. Dev. Biol. 2012;1:693–713. doi: 10.1002/wdev.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Costantini F., Kopan R. Patterning a complex organ: branching morphogenesis and nephron segmentation in kidney development. Dev. Cell. 2010;18:698–712. doi: 10.1016/j.devcel.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saburi S., Hester I., Fischer E., Pontoglio M., Eremina V., Gessler M., Quaggin S.E., Harrison R., Mount R., McNeill H. Loss of Fat4 disrupts PCP signaling and oriented cell division and leads to cystic kidney disease. Nat. Genet. 2008;40:1010–1015. doi: 10.1038/ng.179. [DOI] [PubMed] [Google Scholar]
- 8.Takeuchi S., Takeda K., Oishi I., Nomi M., Ikeya M., Itoh K., Tamura S., Ueda T., Hatta T., Otani H., et al. Mouse Ror2 receptor tyrosine kinase is required for the heart development and limb formation. Genes Cells. 2000;5:71–78. doi: 10.1046/j.1365-2443.2000.00300.x. [DOI] [PubMed] [Google Scholar]
- 9.Lyashenko N., Weissenbock M., Sharir A., Erben R.G., Minami Y., Hartmann C. Mice lacking the orphan receptor ror1 have distinct skeletal abnormalities and are growth retarded. Dev. Dyn. 2010;239:2266–2277. doi: 10.1002/dvdy.22362. [DOI] [PubMed] [Google Scholar]
- 10.Kikuchi A., Yamamoto H., Sato A., Matsumoto S. Wnt5a: its signalling, functions and implication in diseases. Acta Physiol. (Oxf.) 2012;204:17–33. doi: 10.1111/j.1748-1716.2011.02294.x. [DOI] [PubMed] [Google Scholar]
- 11.Yamaguchi T.P., Bradley A., McMahon A.P., Jones S. A Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development. 1999;126:1211–1223. doi: 10.1242/dev.126.6.1211. [DOI] [PubMed] [Google Scholar]
- 12.Cha K.B., Douglas K.R., Potok M.A., Liang H., Jones S.N., Camper S.A. WNT5A signaling affects pituitary gland shape. Mech. Dev. 2004;121:183–194. doi: 10.1016/j.mod.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Cervantes S., Yamaguchi T.P., Hebrok M. Wnt5a is essential for intestinal elongation in mice. Dev. Biol. 2009;326:285–294. doi: 10.1016/j.ydbio.2008.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qian D., Jones C., Rzadzinska A., Mark S., Zhang X., Steel K.P., Dai X., Chen P. Wnt5a functions in planar cell polarity regulation in mice. Dev. Biol. 2007;306:121–133. doi: 10.1016/j.ydbio.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sinha T., Wang B., Evans S., Wynshaw-Boris A., Wang J. Disheveled mediated planar cell polarity signaling is required in the second heart field lineage for outflow tract morphogenesis. Dev. Biol. 2012;370:135–144. doi: 10.1016/j.ydbio.2012.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oishi I., Suzuki H., Onishi N., Takada R., Kani S., Ohkawara B., Koshida I., Suzuki K., Yamada G., Schwabe G.C., et al. The receptor tyrosine kinase Ror2 is involved in non-canonical Wnt5a/JNK signalling pathway. Genes Cells. 2003;8:645–654. doi: 10.1046/j.1365-2443.2003.00662.x. [DOI] [PubMed] [Google Scholar]
- 17.Perantoni A.O., Timofeeva O., Naillat F., Richman C., Pajni-Underwood S., Wilson C., Vainio S., Dove L.F., Lewandoski M. Inactivation of FGF8 in early mesoderm reveals an essential role in kidney development. Development. 2005;132:3859–3871. doi: 10.1242/dev.01945. [DOI] [PubMed] [Google Scholar]
- 18.Grieshammer U., Le M., Plump A.S., Wang F., Tessier-Lavigne M., Martin G.R. SLIT2-mediated ROBO2 signaling restricts kidney induction to a single site. Dev. Cell. 2004;6:709–717. doi: 10.1016/s1534-5807(04)00108-x. [DOI] [PubMed] [Google Scholar]
- 19.Michos O., Goncalves A., Lopez-Rios J., Tiecke E., Naillat F., Beier K., Galli A., Vainio S., Zeller R. Reduction of BMP4 activity by gremlin 1 enables ureteric bud outgrowth and GDNF/WNT11 feedback signalling during kidney branching morphogenesis. Development. 2007;134:2397–2405. doi: 10.1242/dev.02861. [DOI] [PubMed] [Google Scholar]
- 20.Basson M.A., Akbulut S., Watson-Johnson J., Simon R., Carroll T.J., Shakya R., Gross I., Martin G.R., Lufkin T., McMahon A.P., et al. Sprouty1 is a critical regulator of GDNF/RET-mediated kidney induction. Dev. Cell. 2005;8:229–239. doi: 10.1016/j.devcel.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 21.Anderson M.J., Naiche L.A., Wilson C.P., Elder C., Swing D.A., Lewandoski M. TCreERT2, a transgenic mouse line for temporal control of Cre-mediated recombination in lineages emerging from the primitive streak or tail bud. PLoS ONE. 2013;8:e62479. doi: 10.1371/journal.pone.0062479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Amerongen R., Fuerer C., Mizutani M., Nusse R. Wnt5a can both activate and repress Wnt/beta-catenin signaling during mouse embryonic development. Dev. Biol. 2012;369:101–114. doi: 10.1016/j.ydbio.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Najdi R., Proffitt K., Sprowl S., Kaur S., Yu J., Covey T.M., Virshup D.M., Waterman M.L. A uniform human Wnt expression library reveals a shared secretory pathway and unique signaling activities. Differentiation. 2012;84:203–213. doi: 10.1016/j.diff.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakaya M.A., Biris K., Tsukiyama T., Jaime S., Rawls J.A., Yamaguchi T.P. Wnt3a links left-right determination with segmentation and anteroposterior axis elongation. Development. 2005;132:5425–5436. doi: 10.1242/dev.02149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Afzal A.R., Rajab A., Fenske C.D., Oldridge M., Elanko N., Ternes-Pereira E., Tuysuz B., Murday V.A., Patton M.A., Wilkie A.O., et al. Recessive Robinow syndrome, allelic to dominant brachydactyly type B, is caused by mutation of ROR2. Nat. Genet. 2000;25:419–422. doi: 10.1038/78107. [DOI] [PubMed] [Google Scholar]
- 26.Person A.D., Beiraghi S., Sieben C.M., Hermanson S., Neumann A.N., Robu M.E., Schleiffarth J.R., Billington C.J., Jr, van Bokhoven H., Hoogeboom J.M., et al. WNT5A mutations in patients with autosomal dominant Robinow syndrome. Dev. Dyn. 2010;239:327–337. doi: 10.1002/dvdy.22156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patton M.A., Afzal A.R. Robinow syndrome. J. Med. Genet. 2002;39:305–310. doi: 10.1136/jmg.39.5.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao B., Song H., Bishop K., Elliot G., Garrett L., English M.A., Andre P., Robinson J., Sood R., Minami Y., et al. Wnt signaling gradients establish planar cell polarity by inducing Vangl2 phosphorylation through Ror2. Dev. Cell. 2011;20:163–176. doi: 10.1016/j.devcel.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gray R.S., Roszko I., Solnica-Krezel L. Planar cell polarity: coordinating morphogenetic cell behaviors with embryonic polarity. Dev. Cell. 2011;21:120–133. doi: 10.1016/j.devcel.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nishita M., Qiao S., Miyamoto M., Okinaka Y., Yamada M., Hashimoto R., Iijima K., Otani H., Hartmann C., Nishinakamura R., et al. Role of Wnt5a-Ror2 signaling in morphogenesis of the metanephric mesenchyme during ureteric budding. Mol. Cell. Biol. 2014;34:3096–3105. doi: 10.1128/MCB.00491-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miyoshi H., Ajima R., Luo C.T., Yamaguchi T.P., Stappenbeck T.S. Wnt5a potentiates TGF-beta signaling to promote colonic crypt regeneration after tissue injury. Science. 2012;338:108–113. doi: 10.1126/science.1223821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chi X., Hadjantonakis A.K., Wu Z., Hyink D., Costantini F. A transgenic mouse that reveals cell shape and arrangement during ureteric bud branching. Genesis. 2009;47:61–66. doi: 10.1002/dvg.20452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kitagaki J., Ueda Y., Chi X., Sharma N., Elder C.M., Truffer E., Costantini F., Lewandoski M., Perantoni A.O. FGF8 is essential for formation of the ductal system in the male reproductive tract. Development. 2011;138:5369–5378. doi: 10.1242/dev.051888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nguyen M.T., Zhu J., Nakamura E., Bao X., Mackem S. Tamoxifen-dependent, inducible Hoxb6CreERT recombinase function in lateral plate and limb mesoderm, CNS isthmic organizer, posterior trunk neural crest, hindgut, and tailbud. Dev. Dyn. 2009;238:467–474. doi: 10.1002/dvdy.21846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang H., Yang Y., Sharma N., Tarasova N.I., Timofeeva O.A., Winkler-Pickett R.T., Tanigawa S., Perantoni A.O. STAT1 activation regulates proliferation and differentiation of renal progenitors. Cell. Signal. 2010;22:1717–1726. doi: 10.1016/j.cellsig.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yun K., Choi Y.D., Nam J.H., Park Z., Im S.H. NF-kappaB regulates Lef1 gene expression in chondrocytes. Biochem. Biophys. Res. Commun. 2007;357:589–595. doi: 10.1016/j.bbrc.2007.03.170. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.