Abstract
Background.
Age-associated neural changes profoundly affect the biomechanics and energetics of walking, increase energy cost, and require novel approaches to exercise that focus on motor learning theory.
Methods.
We present a conceptual framework for motor skill in walking, its effect on the energy cost of walking, and the influence of the aging brain.
Results.
Motor learning theory and practice can be incorporated into interventions to promote skilled, energy efficient walking in older people.
Conclusions.
An extensive literature on motor skill and motor learning, derived from neuroscience, sports medicine, and neurorehabilitation, can be applied to problems of walking in late life.
Key Words: Brain aging, Motor control, Energy cost of walking, Gait.
Exercise, whether part of a preventive wellness program or restorative therapy, generally consists of activities designed to promote strength, endurance, flexibility, and sometimes balance. Although such approaches can increase mobility, their impact is only modest, with gains of up to 13% in average walking speed (1–4). Greater gains in mobility may potentially be achieved by incorporating motor skill training. Walking is a motor skill, acquired through motor learning. Motor learning leads to functional reorganization of brain activity that generates the preprogrammed neural circuitry required for efficient, automatic walking. Age-associated brain changes can disrupt the neural circuitry of walking and lead to reduced motor skill, loss of automaticity, and gait inefficiency. We will present a conceptual framework for walking as a learned motor skill affecting the energy cost of walking, address the effects of aging and disease on motor skill and energy cost, and consider how motor learning concepts can inform novel interventions to promote mobility in older people.
Walking as a Learned Motor Skill Affecting the Energy Cost of Walking
Motor skill is movement that is smooth, efficient, and automatic. Skilled movement requires minimal attention to the individual components of the action, is goal-oriented, and learned through practice that proceeds through defined stages. Repeated practice results in progressive mastery of longer and more complex movement sequences and is based on iterative modifications, approximations, and adaptations that lead toward more precise and efficient accomplishment of the goal (5–9). The learning and mastery of skilled movement results in a motor behavior that is preplanned, with extensively refined and organized elements, that is more spatially and temporally accurate, performed more precisely, more quickly and with less work or energy (8,10). It is this preplanning that permits a nearly automatic performance of a well-learned, skilled motor task (Table 1). With increasing mastery, the skilled motor task is accomplished with fewer muscles, lower muscle activation amplitudes and durations, more precise sequencing, and closely linked phases of movement acceleration and deceleration, resulting in a smooth velocity profile (5,8).
Table 1.
Characteristics of Novice and Skilled Motor Actions
| Novice Movement | Skilled Movement |
|---|---|
| Peripheral factors | |
| Multiple muscles activated in a prolonged cocontraction pattern | Multiple muscles activated sequentially in brief bursts |
| Variable movement sequence | Preplanned motor sequence |
| Movement subsegments, with stops and starts that redirect to the movement target | Movement acceleration and deceleration is smooth and programmed together |
| Guided, discontinuous movement with an irregular velocity profile | Nonguided, continuous movement with a smooth velocity profile |
| Task-oriented practice is needed to acquire a new motor sequence | Practice necessary is needed to maintain motor expertise and automaticity |
| Central factors | |
| Brain activity in a frontoparietal (cortico-cortico) circuits | Brain activity in cortico-basal ganglia, cortico-cerebellar circuits |
| Sustained, generalized pattern of brain activity | Brief, specific “efficient” pattern of brain activity |
| High cingulate motor area activity | Reduced cingulate motor area activity |
When first attempting a new skill, substantial attention is required to consciously select and guide each aspect of the movement. As skill develops, less attention is required and the movements become more automatic (9,11). Brain activity evolves as skill develops. At first, the brain tends to activate several large regions over both hemispheres (12,13). Early in learning, frontal (eg, prefrontal and premotor) and parietal association areas (eg, posterior and inferior parietal) are typically most active in a cortico-cortico pattern of brain activation (12). This dominantly frontoparietal pattern of brain activation reflects the demand for conscious attention from the learner. As skill develops, brain activity tends to decrease and become more synchronous and localized within motor- and reward-related brain regions (7,12). With greater acquisition of skill, the pattern of brain activation changes from cortico-cortico to a concise neural network of cortico-basal ganglia, which are linked predominantly to the production of skilled motor actions, and cortico-cerebellar circuits, which are associated with the ability to adjust and adapt the preplanned motor skill program to current conditions (Supplementary Appendix Figure A1; 7,14). Milton and coworkers (8) suggested that compared with the novice, the expert shows reduced limbic (cingulate motor area) brain activation, reflecting greater automaticity of movement and less reliance on intentionally, guided actions.
To obtain or maintain motor skill, practice is essential. For novices, practice helps build the motor program, whereas for experts, practice helps sustain the program (8). Feedback during practice is important for learning and leads to self-regulation and automaticity. Automaticity develops as the individual constructs and applies “internal maps” within the brain that characterize the self and the environment, freeing brain resources, and energy (9). Expert movers can regain skill more rapidly than novices can initially create it (8).
Walking is a highly skilled motor task designed to smoothly translate the body over space. Walking expertise develops gradually; toddler’s steps are irregular and noncontinuous, whereas adult’s steps tend to be smooth and continuous. Walking is more than stepping; walking integrates the locomotor stepping pattern (specific sequences of brief bursts of activity in multiple limb muscles) with the cyclic biomechanical phases of gait and with postural demands required to remain upright. The neural control of bipedal walking evolved to coordinate the timing of stepping with the appropriate trunk and limb postures and phase of gait and to modulate postural reflexes, so that the gait pattern is reproducible, adaptable, and efficient (15–17).
Although typical gait characteristics help represent speed and accuracy of walking, they may be less able to capture the integration of stepping with postural adjustments and automaticity. A recent approach is to characterize the “smoothness of walking,” which uses the harmonic ratio to capture cycles of body acceleration in three planes (18). Smoother walking tends to be more energy efficient.
Energy efficient gait is captured through the energy cost of walking. It is a measure of the rate of physiological work during walking (mean oxygen consumption at steady state), standardized by the workload (mean stable gait speed on a defined surface grade) (Supplementary Appendix Table A1; (19,20).
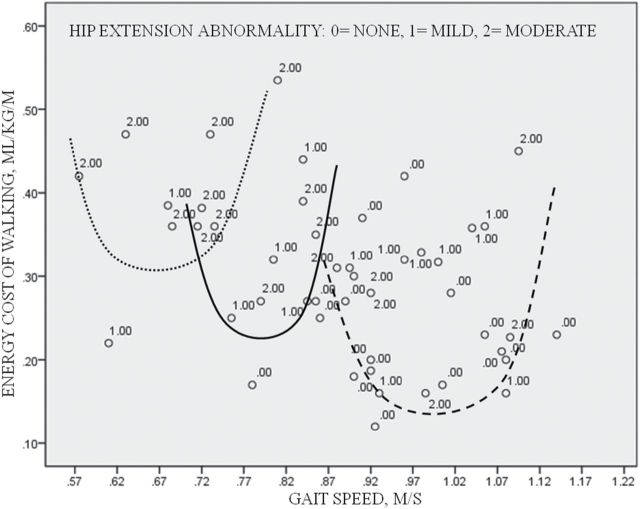
Most healthy individuals have a preferred walking speed that minimizes their energy cost of walking. As an individual varies his gait speed from slow to fast, the energy-speed relation is a “J-curve” (21), concave upward with the lowest energy cost associated with preferred gait speed. In individuals with gait abnormalities, this J-curve relationship can be shifted upward or to the left (22), but preferred gait speed remains at or near minimal energy cost (Figure 1). The neurophysiological mechanisms underlying the energy expenditure–speed relation is still unknown, but the relation highlights the importance of gait efficiency for the organism (21). The energy cost of walking is influenced by many aspects of gait, and gait efficiency in turn affects functional capacity and physical activity (20). Energy cost is not the same as fitness. Fitness is a measure of the capacity to do work, while energy cost reflects the work required by a specific task.
Figure 1.
Shifts in the J-curve of the energy cost–speed relation. Within an individual, the preferred gait speed relates to the lowest energy. More abnormal gait shifts the J-curve relation upward and to the left, but for the abnormal gait, the energy cost of walking remains lowest at a preferred gait speed. Within each “J-curve,” those with the greatest hip extension abnormality tend to have the highest energy cost and walk at gait speeds below or above preferred speed. Visual approximates of J-curves for energy cost-speed relations among older adults with abnormal gait who walked: very slow, dotted line; moderately slow, solid line; and slow, dashed line.
Dickinson and coworkers (16), in describing the science of movement control, suggest that walking is explained by four key factors: (a) muscles performing functions in the context of walking rather than in isolated contractions; (b) mechanisms of energy exchange and use of force for propulsion, stability, and maneuvering; (c) distributed neural control, using feed forward and neural-mechanical feedback; and (d) environmentally induced tradeoffs between intended and appropriate behaviors. The first two represent biomechanical factors such as the use of momentum, and the modulation of acceleration and deceleration using interactive muscle forces and energy transfer. Muscle forces are minimized by the timing of interaction of the limbs with the ground and by stretch response characteristics of limb tissues. Timing is critical for the demands on muscles during walking. The preload (posterolateral shift of the center of pressure of the body over the limb during swing preparation) minimizes the duration of muscle forces needed to generate forward momentum for step initiation toward the stance limb (Supplementary Appendix Figure A2; 23). The coordinated timing of limb loading with the acceleration of the stance limb into extension stores potential energy in the tissues of the anterior thigh during stance, which is then released during swing and “pays” the energy cost of forward translation of the limb (body; 15). The third movement factor reflects neuromuscular factors such as the pattern of muscle activation and neural control (bidirectional feedback between the brain and the periphery; Table 2). The motor pattern for normal human stepping has evolved to override segmental reflex patterns, so that there is a preparatory motor response with inhibition of some muscle groups prior to gait initiation (25). The “mechanical preflex” during hip extension elicits stepping, and the resistance to stretch elicits a rebound response which is quicker that the fastest neural reflex (15,16). These biomechanical and neuromuscular factors interact constantly during gait; the integration saves neural and muscle energy (Supplementary Appendix Table A2). For example, the extensor movement of the loaded stance limb (biomechanical factor) helps generate the signal for stepping (neuromuscular factor). Such an integrated model implies that walking is better explained as an inverted pendulum than by the actions of individual muscles and joints (30).
Table 2.
Age-Related Biomechanical and Neuromuscular Factors Contribute to a Loss of Motor Skill and an Increased Energy Cost of Walking for Older Adults
| Biomechanical Factors | Neuromuscular Factors | ||
|---|---|---|---|
| Use of momentum | Moderating acceleration and deceleration | Pattern of peripheral muscle activation | Neural control |
| Insufficient loading of the limb transitioning to swing for gait initiation (23) | Heel strike poorly timed with push off (24) | Lack of inhibition of antagonist prior to agonist activation (23); excessive muscle activity at gait initiation (25) | Reduced (elicited) signal for stepping – lack of hip extension and loss of contribution of mechanical preflexes (15,16) |
| Stance limb not loaded through midstance in preparation for push off (15,26) | Large vertical displacement of the center of mass (15); reduced plantarflexor power (26) | Prolonged cocontraction of lower limb muscles (27); prolonged contraction and relaxation time (27) | Poor modulation of postural reflexes during the transition from standing to walking (loss of preflex) (15) |
| Trunk flexion (28), limited hip extension (26,28), and reduced ankle dorsiflexion (26) | Disrupted inverted pendulum (24); step width increased (26) | Trunk leading strategy (23,26); instability – additional corrective postural responses (23) | Slowness of movement Intentional guiding of limb movements – placing, step length (26) |
| Inefficient mitochondrial function; de-energized muscle cell (29) | |||
The last of Dickinson’s explanatory factors focuses on environmentally induced tradeoffs when walking in real world conditions. The brain’s role has been likened to sending suggestions to modify behavior of the interactive peripheral neuromechanical/environmental system for gait in the face of changing intent or task demands (15,16). For example, most people walk differently on slippery surfaces like ice, more slowly, with shorter steps and a flat contact of the foot with the surface. Thus, the brain can choose to increase stability and safety but at the cost of a loss of walking energy efficiency.
Effects of Aging and Disease on Motor Skill and Energy Cost
Many age-related changes contribute to increased energy cost of walking, which can be two to four times that of a healthy adult (20,22,26,31,32). Age-related biomechanical factors, such as flexed trunk, limited hip extension, and reduced ankle motion in gait (28), alter normal pendulum actions, resulting in less use of passive stored energy and more demand on muscle activity (24). Age-related neuromuscular factors alter the efficient pattern of recruitment of muscles and the timing of limb movements (Figure 2; 26,27). These age-related biomechanical and neuromuscular factors can create a vicious cycle of increasing gait inefficiency because the compensatory strategies themselves require increased energy (Supplementary Appendix Table A3). Age-related changes in movement speed affect energy cost less specifically but can be pervasive and influential (32–34). Slowness itself interferes with integrated timing of gait which can dramatically reduce gait efficiency (28,32).
Figure 2.
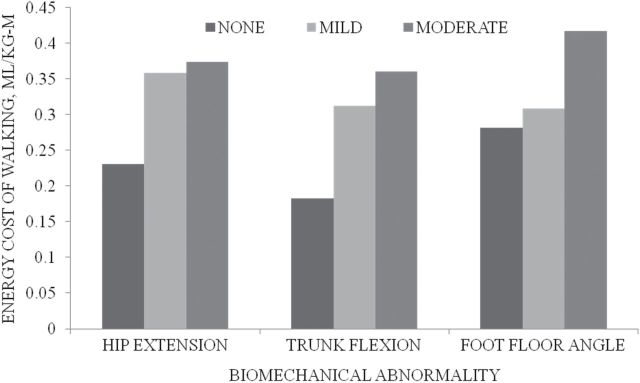
Energy cost of gait abnormalities. The greater the biomechanical abnormality of hip extension, trunk flexion and foot-floor angle, the greater the energy cost of walking.
The loss of motor skill that often accompanies aging leads to reduced automaticity and energy inefficiency and can resemble a more novice-like state of motor skill. Factors that together and separately are associated with loss of motor skill include reduced practice, increased intentional control, and changes in internal maps (35,36). As internal maps degrade without practice, automaticity, which depends on the quality and appropriate selection of internal maps, also degrades. A loss of automaticity leads the individual to perceive increasing gaps between actual and desired motor performance (10), which in turn precipitates more intentionally guided movements. Internal maps must be revised when age or disease alters the central or peripheral body systems involved in movement. To update and rebuild these internal maps, the individual must undertake repeated trial and error practice to help refine movement (10,37,38). However, with age and disease, movement practice can seem risky, and individuals may prefer to solve the problem with increasing intentional control rather than undertake the effort to regain automaticity.
Age-related changes in brain function help explain the loss of motor skill in walking. While damage to specific cortical and subcortical brain structures, as with stroke or Parkinson’s disease, may present with clearly recognizable altered movement patterns (36,38), there is increasing evidence that age-related diffuse processes involving cortical association areas (prefrontal, posterior parietal), the striatum (putamen; 39,40), the cerebellum, and interregional connecting tracts can alter the timing and coordination of movement (41) and are associated with slower walking and balance difficulty in older adults (42,43). Emerging reports from functional neuroimaging document an age-related reduction in efficient patterns of brain activity during motor task performance. Both simple and complex motor tasks in older compared with younger adults have been associated with greater task-related brain activation and a broader network of activated brain regions (14,34). Age-related altered task performance has been associated with increased local connectivity, but decreased distal connectivity in motor-related cortical areas (14). Age-related reductions in brain neurochemistry have been directly and indirectly linked to motor task performance and motor learning problems (6,44). Reduced striatal dopaminergic function was directly associated with altered timing in walking (6,44). While little evidence has directly linked the reduction in cholinergic function with age-related difficulties in motor learning, both the interneuron cholinergic system within the basal ganglia and the cholinergic projection neurons from the basal forebrain nuclei and from the pontine tegmental nuclei have roles in motor skill acquisition and goal-directed learning (45,46). These brain changes can lead to a loss of automaticity and a slow, variable, and inefficient gait.
Age-related changes in neuromuscular function also help explain the age effect on the energy cost of walking. Older adults typically recruit more motor units to generate force with less mass. However, the muscle force demands of usual walking are not great (27), and increased motor unit recruitment is unlikely to explain the age effect on energy cost. Decreased efficiency of mitochondrial energy production with age is emerging as a potentially important influence on the energy cost of walking (29), but to date, mitochondrial function during walking has not been measured.
Motor Learning Concepts Can Inform Novel Strategies to Promote Mobility in Older People
Traditional interventions to improve walking in older adults increase energy capacity by improving fitness and strength. In contrast, motor skill training can help reduce the energy demands of walking (32). Motor skill training allows the brain to relearn and reintegrate the timing and sequence of movements with the postures and phases of gait. Efficient patterns of brain and neuromuscular activation can restore energy efficiency of movement, make walking feel easier, and might lead to reward-based adaptive changes in the brain which may be sustainable (Figure 3; 7,11,47).
Figure 3.
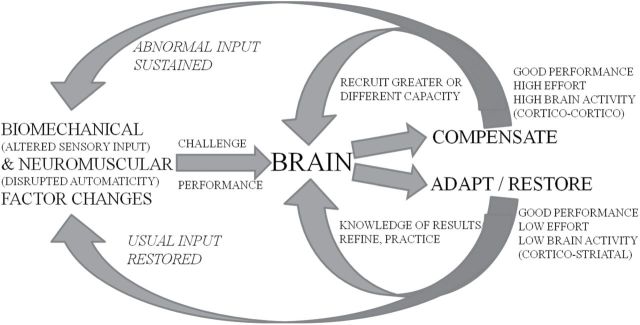
Brain and walking performance: response to challenges. The brain responds to the age-related changes to fix walking performance. Both compensation (use of greater body capacities) and adapt/restore (learn strategies to optimize capacities) can result in good walking performance. Compensation differs from restoration in the both the resources used (effort) and the feedback provided to the brain.
The components of task-oriented motor skill exercise for walking are built from principles of motor skill exercise in sports and neurorehabilitation (5,37,38,48). Motor learning has been shown to be associated with plastic changes in the brain (7), which allow a series of movements to be linked together and become automatic (7,37) and efficient (8,35,47). Characteristics of this approach include: (a) position facilitates the learning of the motor task sequence; (b) exercise is focused on a defined task or goal; (c) task practice is repetitive and increasingly accurate, with or without staged variations in performance conditions or criteria; and (d) feedback knowledge of successful task performance enhances motor skill outcomes (5,34,37). Specific examples are provided in the Supplementary Appendix Table A4. This approach includes both specific task goals and overarching goals that influence the components of exercise and the appropriate outcome assessments. The brain organizes voluntary motor behaviors around an overarching goal, the “motor behavioral goal” (8,49). One concept of a motor behavioral goal of walking is stability and efficiency, in which the nervous system weighs priorities for the motor plans for stability during walking against plans that promote energy efficiency (30). An alternative motor behavioral goal is maneuverability and efficiency, in which the central nervous system prioritizes momentum and efficiency in constructing motor plans (50). Maneuverability refers to the sequences of motor responses to perturbations that first assist or promote the intended action, even at the risk of instability, and are then followed by voluntary movements to restore the path or trajectory of the body (51). The behavioral goal of walking influences how to interpret the intent of the individual, what to measure and the components of the intervention (11,30). The key to successful motor skill exercise is to minimize attention to the components of the task and rather to direct focus to the movement goal of the task. Thus, the performer is engaged in movement problem-solving; the brain is challenged to optimize the motor sequence for the task, which facilitates adaptations to the internal motor map (8,9,11). The outcome of this approach is intended to generate greater automaticity but also may promote the ability to recognize, select, and modify motor programs, leading to a broader repertoire for related movement tasks (5,49) and improvements in daily life functions (32,52,53).
Changes in the aging brain may influence potential response to motor skill training. Age-related reductions in cerebellar norepinephrine were found to be detrimental to motor learning (6). Impaired age-related dopamine function with attendant slowed psychomotor processing, working memory, executive cognitive function, and loss of facilitatory effect on motor sequence learning may contribute to motor learning problems (6). Disrupted connections between cortical areas, white matter disease, and specific disruptions of the integrity of cortical to striatal connections have been associated with difficulty in motor sequence learning (54). These age-related subtle structural and functional brain lesions might interfere with motor skill acquisition by disrupting sensorimotor integration and capacity to reorganize neural networks (6,36). Experiments to assess the effect of age on ability to perform a motor learning task have largely focused on the upper extremity but generally suggest the early phase of motor sequence learning remains intact. Exceptions include practice conditions with unrelated elements or when practice includes explicit information to guide the motor learning task (55). Older adults retain motor sequence learning between practice sessions, but the degree of consolidation is reduced. Older adults adapt the learned motor program more slowly to changed or challenging conditions, yet carryover or transfer of the motor adaptions to similar tasks is not impaired. As a result, older adults may acquire motor skill in walking better if practice is consistent, uses goals and positioning to facilitate “learning by doing” rather than explicit instruction, and involves stage progression over more practice sessions and time.
Gaps, Needs, and Methodological Barriers: Potential Experimental Pathways
Research in the area of motor skill and energy cost can contribute to better ways to prevent and treat mobility decline. Three key areas are: (a) an integrated body-environment concept of walking, (b) mechanisms underlying walking motor behavior, and (c) interventions that reduce compensation and enhance motor skill in walking. For each area, refer to the Supplementary Appendix Table A5 for a list of one or more gaps in knowledge, related needs and methodological barriers, and potential experimental paths. This list is not exhaustive and represents examples of the many potential opportunities. The following paragraphs explore in additional detail some of the ideas listed in the Supplementary Appendix Table A5.
An Integrated Body-Environment Concept of Walking
Walking is an integrated whole body behavior that links intention to interaction with the environment (49). The energy cost of walking influences walking behavior, so energy cost should be assessed and addressed. If energy cost is to be more widely assessed, key methodological issues must be refined, including details of acquisition methods and procedures to estimate energy cost. The integrated model also demands a clearer understanding of the aging brain’s role in responding to the alterations in posture and peripheral neural control that lead to compensated gait. Why isn’t compensated gait corrected spontaneously over time in older adults? Toddlers and young children outgrow inefficient gait patterns through constant practice. In contrast, among older adults with compensated, inefficient gait, an efficient pattern does not develop over time. One way to explore the brain’s role in motor skill and the development of efficient gait is to examine the relationship between brain activity and neuromuscular performance in gait. Using current technology, it is difficult to image whole brain function while people walk. One approach is to use mental imagery (imagined walking) with functional magnetic resonance imaging (56). The major components of brain activation patterns for imagined walking have been shown to be consistent across studies (57). The future of functional magnetic resonance imaging with imagined walking as a window into the efficiency of brain activation will depend on the ability to interpret changes in brain activity associated with changes in motor imagery and practice (58). Another promising approach is to use electroencephalography to record brain activity during walking (59). While the temporal resolution of electroencephalography imaging is useful for capturing the dynamics of brain activity during walking, electroencephalography signal interpretation has been hindered by a poor signal to noise ratio during movement. Because of recent advances in methods to resolve the difficulty of movement related artifacts, electroencephalography may be a useful means to directly examine the relation of brain activity with walking performance (59,60).
Mechanisms Underlying Walking Motor Behavior
Whether the behavioral goal of walking is stability or maneuverability, the goal drives gait assessment and intervention. Standard measures and interventions tend to focus on the components of gait rather than on the behavioral goal of walking. For a stability goal, one might assess gait variability, while for a maneuverability goal, smoothness may be preferred. Some kinds of impairments constrain options for internal maps and affect treatment goals. For example, lack of peripheral sensation greatly reduces feedback during walking, leaving internal maps to create walking behavior based on potentially inaccurate or absent information about the interaction of the limbs, muscle forces, and the ground. At times, stability is preferred over maneuverability due to safety concerns. However, safety and maneuverability are not always incompatible. Maneuverability can promote safety if it increases the individual’s repertoire for walking (49,50).
Optimal Interventions to Reduce Compensation and Enhance Motor Skill in Walking
Motor skill training requires multiple refinements and may be a part of a broader exercise intervention. To increase walking efficiency through motor skill learning, one must consider the motivations and rewards of repeated practice. Walking as a behavior emerges from the integration of performance ability, efficiency, payoff, and intent (called the “affordance competition hypothesis”) (49). The process of motor skill reacquisition may influence payoff and intent, and payoff and intent may be key factors to promote activity and participation. For example, behavior is differentially affected by forced-practice motor training compared with reward-based motor learning (5,6,49). Success can be the reward that sustains a reacquired motor skill (5). If a key to motor skill training is to redevelop internal maps, then artificial tasks like treadmill-assisted gait training, paced-gait training, and walking on straight level paths may fail to generate the internal maps needed for walking in a world of curves, obstacles, uneven surfaces, time limits, and distractions (49). Finally, interventions that increase both energy capacity and efficiency may result in overall better mobility than either intervention alone.
Summary
Concepts of motor skill and gait efficiency have a strong foundation in neuroscience, sports, and neurorehabilitation and should be incorporated into interventions to promote walking. We suggest that motor skill is essential for efficient walking, is often lost with aging, and can potentially be regained through specific types of goal-oriented walking exercise. We propose that by increasing motor skill and decreasing the energy cost, we can make walking easier and more attractive for older people despite a range of health related problems.
Supplementary Material
Supplementary material can be found at: http://biomedgerontology.oxfordjournals.org/
References
- 1. Bean JF, Herman S, Kiely DK, et al. Increased Velocity Exercise Specific to Task (InVEST) training: a pilot study exploring effects on leg power, balance, and mobility in community-dwelling older women. J Am Geriatr Soc. 2004; 52: 799–804. [DOI] [PubMed] [Google Scholar]
- 2. Buchner DM, Cress ME, de Lateur BJ, et al. The effect of strength and endurance training on gait, balance, fall risk, and health services use in community-living older adults. J Gerontol A Biol Sci Med Sci. 1997; 52: M218–M224. [DOI] [PubMed] [Google Scholar]
- 3. LIFE S, Investigators. Effects of a physical activity intervention on measures of physical performance: results of the Lifestyle Interventions and independence for elders pilot (LIFE-P) study. J Gerontol Med Sci. 2006;61A:1157–1165. [DOI] [PubMed] [Google Scholar]
- 4. Wolf SL, O’Grady M, Easley KA, Guo Y, Kressig RW, Kutner M. The influence of intense Tai Chi training on physical performance and hemodynamic outcomes in transitionally frail, older adults. J Gerontol A Biol Sci Med Sci. 2006; 61: 184–189. [DOI] [PubMed] [Google Scholar]
- 5. Brooks V. The Neural Basis of Motor Control. New York: Oxford University Press; 1986. [Google Scholar]
- 6. Seidler RD, Bernard JA, Burutolu TB, et al. Motor control and aging: links to age-related brain structural, functional, and biochemical effects. Neurosci Biobehav Rev. 2010; 34: 721–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Doyon J, Benali H. Reorganization and plasticity in the adult brain during learning of motor skills. Curr Opin Neurobiol. 2005; 15: 161–167. [DOI] [PubMed] [Google Scholar]
- 8. Milton JG, Small SS, Solodkin A. On the road to automatic: dynamic aspects in the development of expertise. J Clin Neurophysiol. 2004; 21: 134–143. [DOI] [PubMed] [Google Scholar]
- 9. Wulf G, Shea C, Lewthwaite R. Motor skill learning and performance: a review of influential factors. Med Educ. 2010; 44: 75–84. [DOI] [PubMed] [Google Scholar]
- 10. Donchin O, Francis JT, Shadmehr R. Quantifying generalization from trial-by-trial behavior of adaptive systems that learn with basis functions: theory and experiments in human motor control. J Neurosci. 2003; 23: 9032–9045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Milton J, Solodkin A, Hlustík P, Small SL. The mind of expert motor performance is cool and focused. Neuroimage. 2007; 35: 804–813. [DOI] [PubMed] [Google Scholar]
- 12. Graydon FX, Friston KJ, Thomas CG, Brooks VB, Menon RS. Learning-related fMRI activation associated with a rotational visuo-motor transformation. Brain Res Cogn Brain Res. 2005; 22: 373–383. [DOI] [PubMed] [Google Scholar]
- 13. Wu T, Chan P, Hallett M. Modifications of the interactions in the motor networks when a movement becomes automatic. J Physiol (Lond). 2008; 586(Pt 17):4295–4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rowe JB, Siebner H, Filipovic SR, et al. Aging is associated with contrasting changes in local and distant cortical connectivity in the human motor system. Neuroimage. 2006; 32: 747–760. [DOI] [PubMed] [Google Scholar]
- 15. Capaday C. The special nature of human walking and its neural control. Trends Neurosci. 2002; 25: 370–376. [DOI] [PubMed] [Google Scholar]
- 16. Dickinson MH, Farley CT, Full RJ, Koehl MA, Kram R, Lehman S. How animals move: an integrative view. Science. 2000; 288: 100–106. [DOI] [PubMed] [Google Scholar]
- 17. Lay BS, Sparrow WA, Hughes KM, O’Dwyer NJ. Practice effects on coordination and control, metabolic energy expenditure, and muscle activation. Hum Mov Sci. 2002; 21: 807–830. [DOI] [PubMed] [Google Scholar]
- 18. Brach JS, McGurl D, Wert D, et al. Validation of a measure of smoothness of walking. J Gerontol A Biol Sci Med Sci. 2011; 66: 136–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Boyd R, Fatone S, Rodda J, et al. High- or low- technology measurements of energy expenditure in clinical gait analysis? Dev Med Child Neurol. 1999; 41: 676–682. [DOI] [PubMed] [Google Scholar]
- 20. Waters R. Energy expenditure. In: Perry J, ed. Gait Analysis: Normal and Pathologic Function. Thorofare, NJ: Slack Inc.; 2004:443–489. [Google Scholar]
- 21. Zarrugh MY, Todd FN, Ralston HJ. Optimization of energy expenditure during level walking. Eur J Appl Physiol Occup Physiol. 1974; 33: 293–306. [DOI] [PubMed] [Google Scholar]
- 22. Martin PE, Rothstein DE, Larish DD. Effects of age and physical activity status on the speed-aerobic demand relationship of walking. J Appl Physiol. 1992; 73: 200–206. [DOI] [PubMed] [Google Scholar]
- 23. Polcyn AF, Lipsitz LA, Kerrigan DC, Collins JJ. Age-related changes in the initiation of gait: degradation of central mechanisms for momentum generation. Arch Phys Med Rehabil. 1998; 79: 1582–1589. [DOI] [PubMed] [Google Scholar]
- 24. Kuo AD, Donelan JM, Ruina A. Energetic consequences of walking like an inverted pendulum: step-to-step transitions. Exerc Sport Sci Rev. 2005; 33: 88–97. [DOI] [PubMed] [Google Scholar]
- 25. Earles D, Vardaxis V, Koceja D. Regulation of motor output between young and elderly subjects. Clin Neurophysiol. 2001; 112: 1273–1279. [DOI] [PubMed] [Google Scholar]
- 26. McGibbon CA. Toward a better understanding of gait changes with age and disablement: neuromuscular adaptation. Exerc Sport Sci Rev. 2003; 31: 102–108. [DOI] [PubMed] [Google Scholar]
- 27. Hortobágyi T, Finch A, Solnik S, Rider P, DeVita P. Association between muscle activation and metabolic cost of walking in young and old adults. J Gerontol A Biol Sci Med Sci. 2011; 66: 541–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wert DM, Brach J, Perera S, VanSwearingen JM. Gait biomechanics, spatial and temporal characteristics, and the energy cost of walking in older adults with impaired mobility. Phys Ther. 2010; 90: 977–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lanza IR, Nair KS. Mitochondrial function as a determinant of life span. Pflugers Arch. 2010; 459: 277–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kuo AD, Donelan JM. Dynamic principles of gait and their clinical implications. Phys Ther. 2010; 90: 157–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Berryman N, Gayda M, Nigam A, Juneau M, Bherer L, Bosquet L. Comparison of the metabolic energy cost of overground and treadmill walking in older adults. Eur J Appl Physiol. 2012; 112: 1613–1620. [DOI] [PubMed] [Google Scholar]
- 32. VanSwearingen JM, Perera S, Brach JS, Cham R, Rosano C, Studenski SA. A randomized trial of two forms of therapeutic activity to improve walking: effect on the energy cost of walking. J Gerontol A Biol Sci Med Sci. 2009; 64: 1190–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Welford AT. Reaction time, speed of performance, and age. Ann N Y Acad Sci. 1988; 515: 1–17. [DOI] [PubMed] [Google Scholar]
- 34. Wu T, Hallett M. The influence of normal human ageing on automatic movements. J Physiol (Lond). 2005; 562(Pt 2):605–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Doyon J, Bellec P, Amsel R, et al. Contributions of the basal ganglia and functionally related brain structures to motor learning. Behav Brain Res. 2009; 199: 61–75. [DOI] [PubMed] [Google Scholar]
- 36. Wu T, Chan P, Hallett M. Effective connectivity of neural networks in automatic movements in Parkinson’s disease. Neuroimage. 2010; 49: 2581–2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cramer SC, Sur M, Dobkin BH, et al. Harnessing neuroplasticity for clinical applications. Brain. 2011; 134(Pt 6):1591–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kleim JA. Neural plasticity and neurorehabilitation: teaching the new brain old tricks. J Commun Disord. 2011; 44: 521–528. [DOI] [PubMed] [Google Scholar]
- 39. Dreher JC, Grafman J. The roles of the cerebellum and basal ganglia in timing and error prediction. Eur J Neurosci. 2002; 16: 1609–1619. [DOI] [PubMed] [Google Scholar]
- 40. Nenadic I, Gaser C, Volz HP, Rammsayer T, Häger F, Sauer H. Processing of temporal information and the basal ganglia: new evidence from fMRI. Exp Brain Res. 2003; 148: 238–246. [DOI] [PubMed] [Google Scholar]
- 41. DeCarli C, Kawas C, Morrison JH, Reuter-Lorenz PA, Sperling RA, Wright CB. Session II: Mechanisms of age-related cognitive change and targets for intervention: neural circuits, networks, and plasticity. J Gerontol A Biol Sci Med Sci. 2012; 67: 747–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rosano C, Aizenstein HJ, Studenski S, Newman AB. A regions-of-interest volumetric analysis of mobility limitations in community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2007; 62: 1048–1055. [DOI] [PubMed] [Google Scholar]
- 43. Baezner H, Blahak C, Poggesi A, et al. LADIS Study Group. Association of gait and balance disorders with age-related white matter changes: the LADIS study. Neurology. 2008; 70: 935–942. [DOI] [PubMed] [Google Scholar]
- 44. Cham R, Studenski SA, Perera S, Bohnen NI. Striatal dopaminergic denervation and gait in healthy adults. Exp Brain Res. 2008; 185: 391–398. [DOI] [PubMed] [Google Scholar]
- 45. Pisani A, Bernardi G, Ding J, Surmeier DJ. Re-emergence of striatal cholinergic interneurons in movement disorders. Trends Neurosci. 2007; 30: 545–553. [DOI] [PubMed] [Google Scholar]
- 46. Schliebs R, Arendt T. The cholinergic system in aging and neuronal degeneration. Behav Brain Res. 2011; 221: 555–563. [DOI] [PubMed] [Google Scholar]
- 47. King BR, Fogel SM, Albouy G, Doyon J. Neural correlates of the age-related changes in motor sequence learning and motor adaptation in older adults. Front Hum Neurosci. 2013; 7: 142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Handford C, Davids K, Bennett S, Button C. Skill acquisition in sport: some applications of an evolving practice ecology. J Sports Sci. 1997; 15: 621–640. [DOI] [PubMed] [Google Scholar]
- 49. Cisek P, Kalaska JF. Neural mechanisms for interacting with a world full of action choices. Annu Rev Neurosci. 2010; 33: 269–298. [DOI] [PubMed] [Google Scholar]
- 50. Huang H, Ahmed A. Tradeoff between stability and maneuverability during whole-body movements. PLoS One. 2011;6:e2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hasan Z. The human motor control system’s response to mechanical perturbation: should it, can it, and does it ensure stability? J Mot Behav. 2005; 37: 484–493. [DOI] [PubMed] [Google Scholar]
- 52. Nadkarni NK, Studenski SA, Perera S, et al. White matter hyperintensities, exercise, and improvement in gait speed: does type of gait rehabilitation matter? J Am Geriatr Soc. 2013; 61: 686–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Van Peppen RP, Kwakkel G, Wood-Dauphinee S, et al. The impact of physical therapy on functional outcomes after stroke: what’s the evidence? Clin Rehabil. 2004;18:833–862. [DOI] [PubMed] [Google Scholar]
- 54. Bennett IJ, Madden DJ, Vaidya CJ, Howard JH, Jr, Howard DV. White matter integrity correlates of implicit sequence learning in healthy aging. Neurobiol Aging. 2011; 32: 2317.e1–2317.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Willingham DB, Salidis J, Gabrieli JD. Direct comparison of neural systems mediating conscious and unconscious skill learning. J Neurophysiol. 2002; 88: 1451–1460. [DOI] [PubMed] [Google Scholar]
- 56. Szameitat AJ, Shen S, Sterr A. Motor imagery of complex everyday movements. An fMRI study. Neuroimage. 2007; 34: 702–713. [DOI] [PubMed] [Google Scholar]
- 57. Godde B, Voelcker-Rehage C. More automation and less cognitive control of imagined walking movements in high- versus low-fit older adults. Front Aging Neurosci. 2010; 2: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Saimpont A, Malouin F, Tousignant B, Jackson PL. Motor imagery and aging. J Mot Behav. 2013; 45: 21–28. [DOI] [PubMed] [Google Scholar]
- 59. Gwin JT, Gramann K, Makeig S, Ferris DP. Removal of movement artifact from high-density EEG recorded during walking and running. J Neurophysiol. 2010; 103: 3526–3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Reis PMR HF, Gabsteiger F, von Tscharner V, Lochmann M. Methodological aspects of EEG and body dynamics measurement during motion. Frontiers Human Neurosci. 2014;8:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.