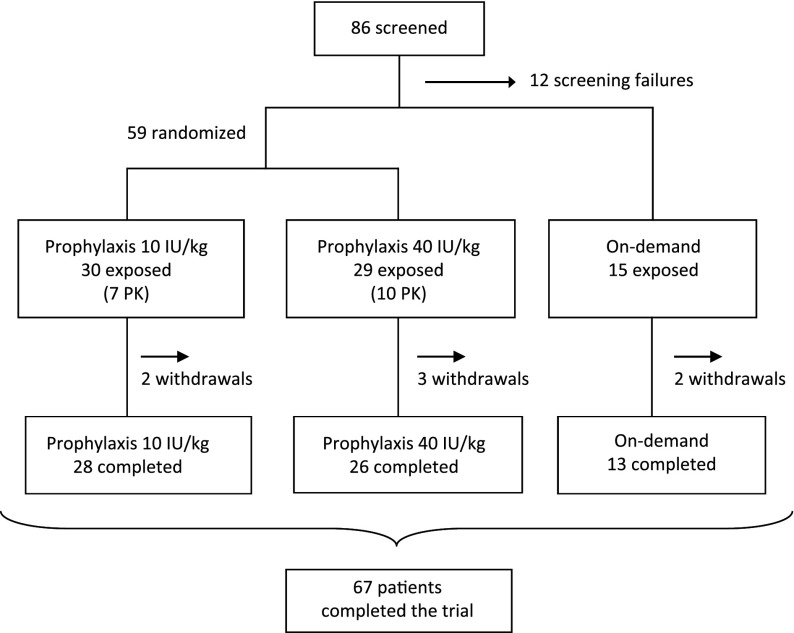
Figure 1.
Patient enrolment and outcomes. A total of 86 patients were screened, of whom 12 were screening failures, leaving 74 patients who were exposed to nonacog beta pegol. At the screening visit, the patient and the investigator decided together whether the patient should be allocated to prophylaxis (59 patients) or on-demand treatment (15 patients). Patients allocated to prophylaxis were randomly assigned to once-weekly dosing of either 10 or 40 IU/kg. A total of 7 patients were withdrawn during the trial, distributed evenly between the treatment groups. None of the withdrawals were a result of adverse events. Screening failures and withdrawals together constituted 19 (22%) of the 86 screened patients. A total of 17 patients participated in a pharmacokinetic session at trial initiation, and all but 1 had a second pharmacokinetic session after 12 to 44 weeks of prophylaxis, leaving 16 patients (7 in the 10 IU/kg group and 9 in the 40 IU/kg group) with complete pharmacokinetic assessments.