Background: BK channels regulate smooth muscle contractility and provide docking site for steroids, such as bile acids.
Results: Non-steroid leukotriene B4 activates the BK channel via a steroid-sensing site.
Conclusion: Physiological lipids from different chemical families share their recognition site in BK proteins.
Significance: Our data underscore a likely cross-talk between different physiological lipids in BK channel-driven pathophysiology.
Keywords: Computer Modeling, Drug Design, Ion Channel, Leukotriene, Patch Clamp
Abstract
Calcium/voltage-gated, large conductance potassium (BK) channels control numerous physiological processes, including myogenic tone. BK channel regulation by direct interaction between lipid and channel protein sites has received increasing attention. Leukotrienes (LTA4, LTB4, LTC4, LTD4, and LTE4) are inflammatory lipid mediators. We performed patch clamp studies in Xenopus oocytes that co-expressed BK channel-forming (cbv1) and accessory β1 subunits cloned from rat cerebral artery myocytes. Leukotrienes were applied at 0.1 nm–10 μm to either leaflet of cell-free membranes at a wide range of [Ca2+]i and voltages. Only LTB4 reversibly increased BK steady-state activity (EC50 = 1 nm; Emax reached at 10 nm), with physiological [Ca2+]i and voltages favoring this activation. Homomeric cbv1 or cbv1-β2 channels were LTB4-resistant. Computational modeling predicted that LTB4 docked onto the cholane steroid-sensing site in the BK β1 transmembrane domain 2 (TM2). Co-application of LTB4 and cholane steroid did not further increase LTB4-induced activation. LTB4 failed to activate β1 subunit-containing channels when β1 carried T169A, A176S, or K179I within the docking site. Co-application of LTB4 with LTA4, LTC4, LTD4, or LTE4 suppressed LTB4-induced activation. Inactive leukotrienes docked onto a portion of the site, probably preventing tight docking of LTB4. In summary, we document the ability of two endogenous lipids from different chemical families to share their site of action on a channel accessory subunit. Thus, cross-talk between leukotrienes and cholane steroids might converge on regulation of smooth muscle contractility via BK β1. Moreover, the identification of LTB4 as a highly potent ligand for BK channels is critical for the future development of β1-specific BK channel activators.
Introduction
Regulation of cell physiology by direct targeting of cell membrane ion channel proteins by lipid signaling molecules represents an area of expanding investigation (1). In particular, lipid regulation of Ca2+- and voltage-gated potassium channels of large conductance (BK)3 has received increasing attention because these channels control numerous physiological processes, such as smooth muscle tone, neuronal firing, hormonal release, immune responses, and cancer cell growth (2–4). Functional BK channels result from tetrameric association of the ubiquitously expressed α (slo1) subunit, which contains an S1–S6 transmembrane (TM) core common to other voltage-gated ion channels of the TM6 superfamily (2). In most tissues, however, BK complexes include small accessory proteins (two TM helices) termed β subunits (2). Four types of β subunits (β1–4) have been identified, with highly distinct and tissue-specific expression profiles (5, 6). Each β-subunit type empowers the BK channel complex with a distinct phenotype, which allows this family of channels to contribute to cell physiology in a tissue-specific manner and opens the possibility to selectively modify tissue function by targeting specific BK β type(s). For example, BK β1 allows the BK channel to activate in response to physiological voltage and [Ca2+]i found in contracting smooth muscle cells. Upon activation, BK channels generate outward potassium currents that oppose membrane depolarization (2). Thus, activation of β1-containing BK channels is a powerful mechanism to evoke smooth muscle relaxation, a desirable end point to treat prevalent human conditions, including bronchial asthma, pulmonary hypertension, overactive bladder, vascular spasm, and arterial hypertension.
Specific sites for lipid modulation have been identified in both slo1 and BK β subunits. For instance, cholesterol regulates BK channel activity by interacting with several cholesterol recognition amino acid consensus motifs located along the slo1 cytosolic tail domain (7). Phosphatidylinositol 4,5-bisphosphate and related phosphoinositides also interact with slo1, yet their recognition site involves an RKK triplet located immediately after S6 (8). In contrast, a diverse group of lipids requires the presence of accessory β subunits within the BK complex for BK current modulation. 17β-Estradiol activates BK channels in the presence of the β1 subunit (9). On the other hand, fatty acid-induced BK channel activation is best observed in the presence of β1 or β4 subunits (10). Finally, cholane steroids increase BK channel activity by specific interaction with a discrete site located in the BK β1 TM2 (11–12). This site is unique to β1 and is not present in other β subunits. In all cases, the lipid effect on BK channel function was observed in cell-free membrane patches. Therefore, a wide variety of physiologically important lipids is likely to alter BK channel activity and, thus, tissue function by selective interaction with distinct sites located in the BK slo1 or β subunits.
Leukotrienes (LTA4, LTB4, LTC4, LTD4, and LTE4) are important inflammatory mediators (derived from arachidonic acid via the 5-lipoxygenase pathway) that exert their physiological effects by activating G protein-coupled receptors (13). Circulating levels of leukotrienes in health and disease do not reach micromolar levels (14–16). The chemical structure of these endogenous lipids includes a long hydrophobic backbone and at least one carboxylic acid end group (Fig. 1). Here we demonstrate that LTB4 activates BK channels by direct interaction with the cholane steroid-sensing site located in the BK β1 TM2. This finding is critical for the future development of β1-specific BK channel activators. Moreover, we demonstrate that LTB4 activates BK channels with an unusually high potency (EC50 = 1 nm). The fact that the EC50 value matches physiologically relevant levels of LTB4 in the vascular system raises speculation that cross-talk between endogenous leukotrienes and cholane steroids may converge to regulate smooth muscle contractility via BK β1 targeting.
FIGURE 1.
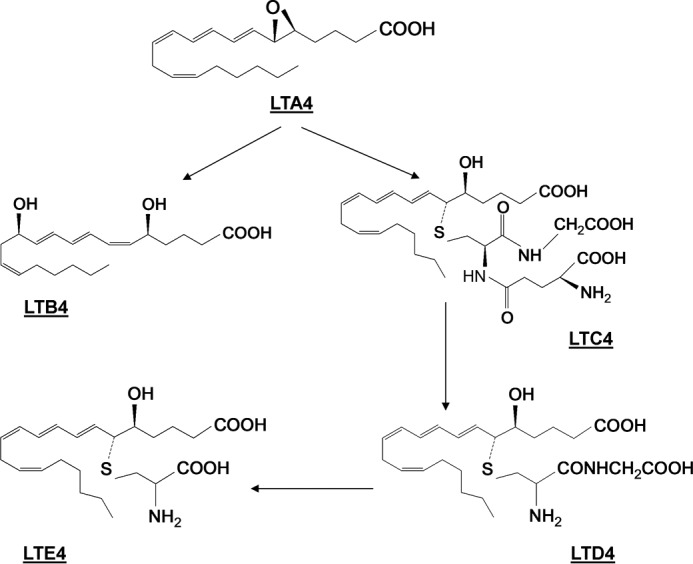
Chemical structure and biosynthetic pathway of the LT4 family of lipids.
MATERIALS AND METHODS
Ethical Aspects of Research
The care of animals and experimental protocols were reviewed and approved by the Animal Care and Use Committee of the University of Tennessee Health Science Center, an institution accredited by the Association for Assessment and Accreditation of Laboratory Animal Care.
cRNA Preparation
Cloning, expression, and functional characterization of cbv1 (AY330293) are described elsewhere (17, 18). β1 cDNA from rat cerebral artery smooth muscle cells (FJ154955) was subcloned into pOX vector and expressed as described (18). Targeted mutations were introduced into rβ1 cDNA by using overlap-extension polymerase chain reaction (PCR) and QuikChange (Stratagene) with Pfu polymerase following the manufacturer's instructions. In the PCR-amplified regions of all constructs, the presence of targeted mutations and the absence of unintended mutations were verified by automated sequencing at the Molecular Resource Center (University of Tennessee Health Science Center). The cDNA coding for the β2 subunit without its N-type inactivation domain (β2IR) (a generous gift from Dr. Ligia Toro; UCLA) was subcloned into pOX vector for expression in oocytes. β1 and β2IR cDNAs inserted into pOX vectors were linearized and then transcribed in vitro by using the mMessage mMachine transcription kit with T3 and T7 polymerase, respectively (Ambion). cRNA was dissolved in diethyl polycarbonate-treated water at 10 (cbv1) and 30 (β1 or β2IR) ng/μl; 1-μl aliquots were stored at −70 °C.
Oocyte Extraction and cRNA Injection
Xenopus laevis females were purchased from Xenopus Express and maintained in artificial pond water on a 12-h light/dark cycle. In this habitat, the females do not show seasonal breeding behavior, so oocytes are available throughout the year. Stage V and VI oocytes were predominantly used because they transcribe mRNA into channels efficiently. Six frogs were used as oocyte donors. Before surgery, the frogs were anesthetized by placement on ice after exposure to ethyl 3-aminobenzoate methanesulfonate salt (250 mg/liter, pH 7.4). Oocytes were removed and kept in Ca2+-free ND-96 solution (82.5 mm NaCl, 2 mm KCl, 1 mm MgCl2, 5 mm HEPES, pH 7.5) containing 2 mg/ml type IV collagenase (Sigma-Aldrich) in a shaker (60 oscillations/min) at room temperature for 15 min to remove the follicular layer. After defolliculation, oocytes were transferred to Ca2+-containing ND-96 saline (82.5 mm NaCl, 2 mm KCl, 1.8 mm CaCl2, 5 mm HEPES, pH 7.4), supplemented with 2.5 mm sodium pyruvate and 2 mg/ml gentamicin. Injection of mRNA into the oocyte cytoplasm was conducted using a Drummond micropipette modified for microinjection (Drummond Scientific Co.). The interval between cRNA injection and patch clamping was 36–48 h, during which time oocytes were kept at 15 °C.
Electrophysiology Data Acquisition and Analysis
Before electrophysiological recording, oocytes were placed into a dish containing a hypertonic solution (200 mm K+ aspartate, 20 mm KCl, 1 mm MgCl2, 10 mm EGTA, 10 mm HEPES, pH 7.4) for 10 min. With this treatment, the oocytes shrank, allowing the removal of the vitelline layer with forceps and exposing the oocyte membrane for subsequent patch clamp recording. Then oocytes were placed back into ND-96 saline (in this case without gentamicin; for the composition, see above) for 10–15 min before ionic current recording. Unless stated otherwise, currents were recorded from excised, inside-out (I/O) patches. Both bath and electrode solutions contained 130 mm K+ gluconate, 5 mm EGTA, 2.28 mm MgCl2, 15 mm HEPES, and 1.6 mm HEDTA, pH 7.35. In all experiments, the free [Ca2+] in solution was adjusted to the desired value by adding CaCl2. When the desired free [Ca2+] did not exceed 1 μm, HEDTA was omitted from the solution, and the final MgCl2 was set to 1 mm. In all cases, the nominal free [Ca2+] was calculated with MaxChelator Sliders.
Patch clamp electrodes were pulled from glass capillaries (Drummond Scientific Co.). Immediately before recording, the tip of the electrode was fire-polished on a microforge (World Precision Instruments) to give resistances of 8–10 megaohms when filled with extracellular solution (for composition, see above). An agar bridge with gluconate as main anion was used as the ground electrode. After excision from the cell, the membrane patch was exposed to a stream of bath solution containing each agent at final concentration. Solutions were applied onto the patch cytosolic side by using a pressurized, automated OctaFlow perfusion system (ALA Scientific Instruments) via a micropipette tip with an internal diameter of 100 μm. Experiments were performed at room temperature (20–22 °C).
Ionic current was recorded using an EPC8 amplifier (HEKA, Lambrecht, Germany) at 1 kHz. Data were digitized at 5 kHz by using a Digidata 1320A A/D converter and pCLAMP version 8.0 (Molecular Devices).
Computational Modeling
The leukotriene molecular structures were built using the Molecular Operating Environment 2013.08 software package (Chemical Computing Group) with the carboxylic acid in the negatively charged form expected at physiological pH. The structures were minimized using the MMFF94x force field. A flexible superposition was performed with lithocholic acid (LCA) and leukotriene B4 using default settings in Molecular Operating Environment. The previously described model of the β1 TM2 (15) was used to dock all leukotrienes on the steroid-sensing site. The Induced Fit docking protocol in Molecular Operating Environment was used, and residues Thr-169 and Lys-179 were selected as the receptor site for the simulation. All other options were left at default settings.
Chemicals
LTA4 methyl ester, LTB4, LTC4, LTD4, and LTE4 were purchased from Cayman Chemical Co. All other chemicals were purchased from Sigma-Aldrich. Immediately before experiments, leukotriene A4 methyl ester was hydrolyzed to render LTA4 following the manufacturer's instructions. Because LTB4, LTC4, LTD4, and LTE4 were supplied in ethanol solution, on the day of experiments, an LT-containing aliquot was dried under an N2 atmosphere to evaporate ethanol. The remaining leukotriene was resuspended in patch clamp recording solution to final concentration.
Data Analysis
For patch clamp recording at single-channel resolution, we used NPo as the index of channel steady-state activity, with NPo being the product of the number of channels in the patch (N) and the individual channel open probability (Po). NPo was obtained using a built-in function in Clampfit version 9.2 software (Molecular Devices) (19, 20). NPo was obtained from 1 min of gap-free recording under each condition.
Final plotting, fitting, and statistical analysis of data were conducted using Origin version 8.5.1 (OriginLab) and InStat version 3.0 (GraphPad). Statistical analysis was conducted using either one-way analysis of variance and Bonferroni's multiple comparison test or paired Student's t test, according to the experimental design. Statistical significance was set at p < 0.05. Data are expressed as mean ± S.E., and n = number of patches. Each patch was obtained from a different oocyte.
RESULTS
LTB4, but Not Other Leukotrienes of the LT4 Family, Activated β1 Subunit-containing BK Channels
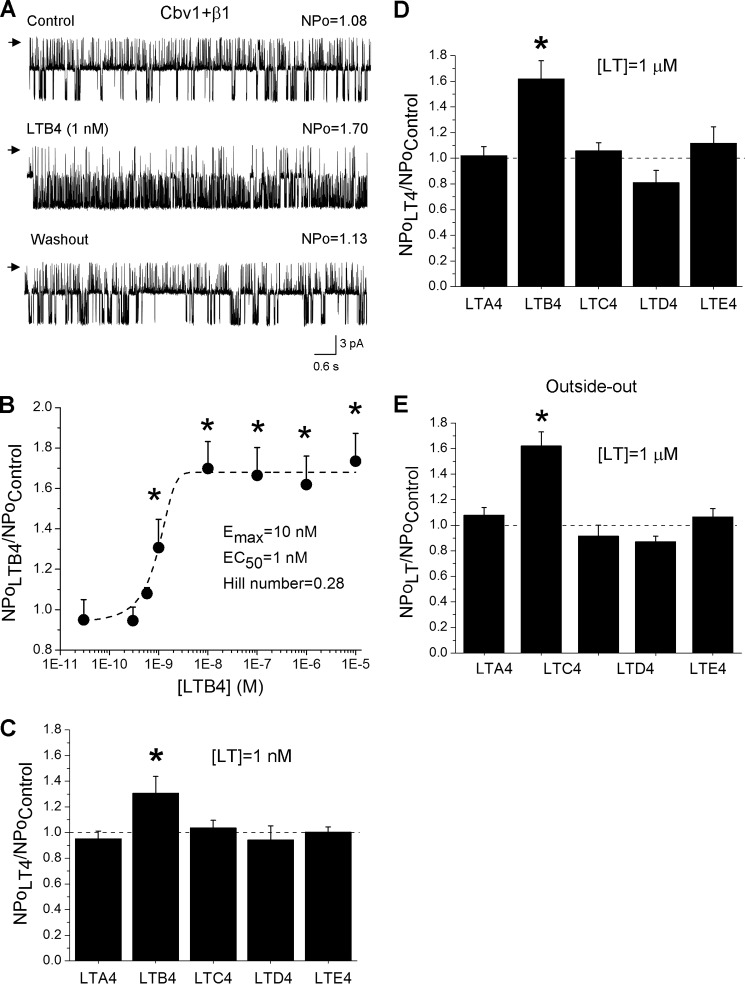
To determine the leukotriene ability to modulate BK channel function, we tested leukotriene action on recombinant channels heterologously expressed in Xenopus oocytes. This expression system has been extensively validated by us and by others to study basic function and lipid modulation of recombinant channels with a subunit composition that matches that of the native smooth muscle BK protein complex (8, 11, 21, 22): BK channel-forming α (“cbv1”; AY330293) and accessory β1 (FJ154955) subunits from freshly isolated rat cerebral artery myocytes. The resulting recombinant BK complex represents an ideal model of the native β1 subunit-containing BK channel (18). We used I/O patches with the membrane potential and free [Ca2+]i set at values that were close to those reported in the rat cerebrovascular myocyte during contraction (−40 to −25 mV and 10 μm). Under these physiological conditions, BK channel activity plays a critical role in limiting depolarization-induced Ca2+ entry and thus opposes smooth muscle contraction (23–25). After excision, each patch was exposed to control solution, and BK activity was continuously recorded for 1 min. The presence of functional β1 subunits within the cbv1-β1 complex was confirmed by the characteristic decrease in voltage that was needed to reach half-maximal current (Vhalf): 20 ± 2.7 and −20 ± 1.7 mV for cbv1 and cbv1-β1, respectively. This macroscopic current phenotype matched what was previously described by us and others (5, 18, 26).
Application of the LTB4-containing (0.1 nm to 10 μm) solution reversibly increased cbv1-β1 NPo up to 2-fold in a concentration-dependent manner: EC50 ∼1 nm, with Emax being reached at ∼10 nm (Fig. 2, A and B). However, other members of the LT4 family (LTA4, LTC4, LTD4, and LTE4) were ineffective in changing cbv1-β1 NPo even when tested at 1 μm, which is 10-fold more than needed to invoke Emax by LTB4 (Fig. 2, C and D). This lack of efficacy of LTA4, LTC4, LTD4, and LTE4 could potentially arise from the difficulty that polar/charged lipids could have in reaching a site located inside the bilayer or in the vicinity of the extracellular side of the membrane patch when the polar lipid accesses the membrane from its cytosolic side. Thus, we tested leukotriene's effect on cbv1-β1 complexes in outside-out (O/O) patches, where agents of interest were perfused onto the extracellular side of the membrane. In O/O patches, the LTB4-induced cbv1-β1 channel activation was similar to that observed in I/O patches (Fig. 2E). Also consistent with data from I/O patches, LTA4, LTC4, LTD4, and LTE4 (1 μm) all failed to increase cbv1-β1 NPo when applied to the extracellular side of O/O patches (Fig. 2E). Therefore, among the LT4 lipids tested, LTB4 was the only one that effectively activated cbv1-β1 channels, this action being independent of the membrane leaflet from which the leukotriene accesses the channel complex.
FIGURE 2.
Leukotriene B4, but not other leukotrienes of the LT4 family, activates recombinant (cbv1-β1) BK channel. A, single-channel recordings from I/O patches excised from X. laevis oocytes expressing cbv1-β1 channel complexes. Current records depict cbv1-β1 channel activity before, during, and after 1 nm LTB4 application. Vm = −20 mV; [Ca2+]i = 10 μm. Here and in all other figures, channel openings are shown as downward deflections; arrows indicate the baseline (all channels closed); control and washout correspond to bath solution. Unless stated otherwise, all data were obtained using the I/O patch configuration. B, concentration response curve for LTB4. Each point represents the average of no fewer than three patches, each patch obtained from a different oocyte. C, averaged increase in cbv1-β1 channel activity (NPo) by different leukotrienes applied at 1 nm. D, averaged increase in cbv1-β1 channel NPo by different leukotrienes applied at 1 μm. E, averaged increase in cbv1-β1 channel NPo by different leukotrienes applied at 1 μm to the extracellular side of the patch in the O/O patch configuration. In C–E, an asterisk indicates difference from control (p < 0.05). Error bars, S.E.
To gain additional insight into the LTB4-induced BK channel activation, we addressed how lipid action related to basal channel activity and its voltage and Ca2+ dependence. The LTB4-induced increase in BK channel activity inversely correlated with basal channel activity (i.e. the channel's Po before LTB4 application) (Fig. 3A). This is somewhat expected for most ion channel activators, because channels with a basal (i.e. pre-agonist) open probability that gets closer to 1 cannot be opened much more (ceiling effect). Next, we explored a possible relationship between the LTB4-induced increase in BK channel activity and the voltage and Ca2+ dependence of BK NPo. The LTB4-induced increase in BK NPo correlated poorly with transmembrane voltage (Fig. 3B). On the other hand, a plot of LTB4-induced BK channel activation versus [Ca2+]i exhibited a complex shape (Fig. 3C). First, LTB4 failed to activate the BK channel in the absence of [Ca2+]i and at submicromolar [Ca2+]i. This finding is consistent with β1 subunit-dependent mechanisms for LTB4 effect; at submicromolar [Ca2+]i, β1 subunit regulation of channel activity is not apparent from Po evaluation (27). Second, low micromolar [Ca2+]i seemed optimal for LTB4-induced increase in BK NPo. Finally, LTB4-induced BK channel activation vanished when [Ca2+]i reached 30 μm. One plausible explanation for this loss of activity is that at high levels of activating [Ca2+]i, BK Po becomes too high for effective LTB4-induced channel activation.
FIGURE 3.
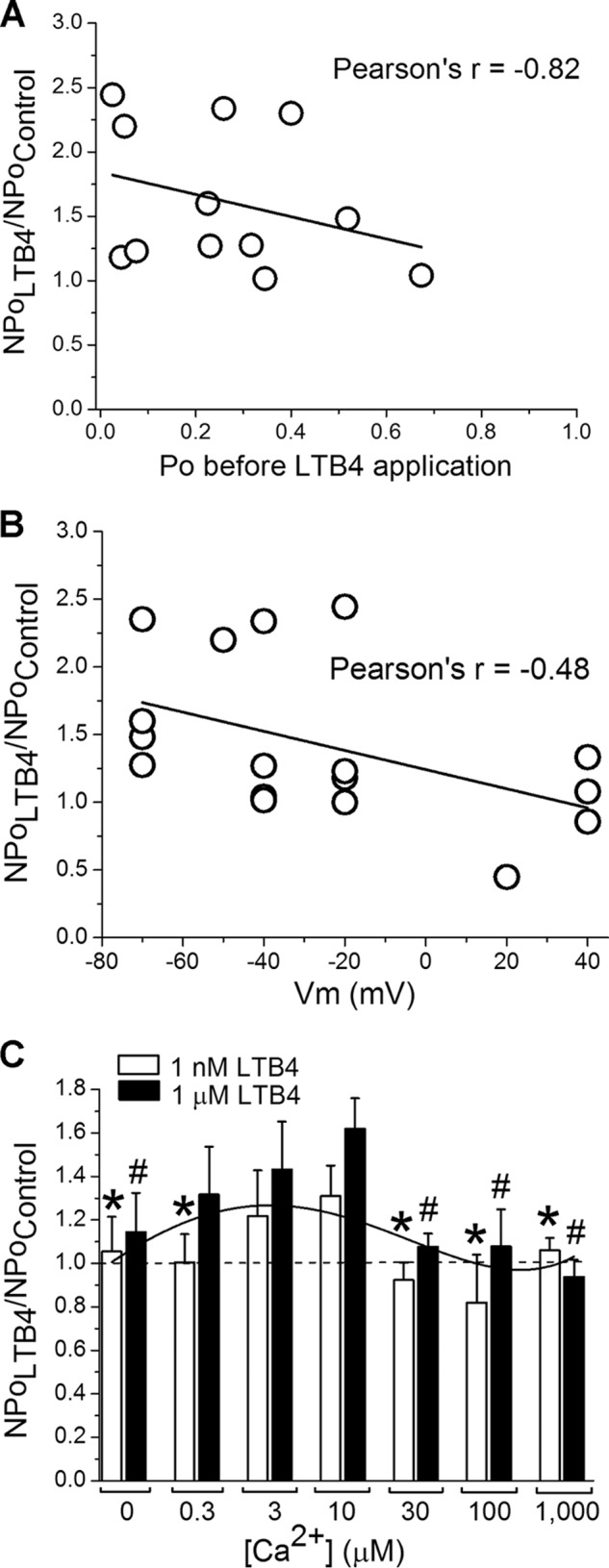
Biophysical characteristics of LTB4-induced increase in cbv1-β1 channel activity. A, LTB4-induced increase in cbv1-β1 channel's NPo shows strong negative correlation with basal channel activity (Po before LTB4 application). B, LTB4-induced increase in cbv1-β1 channel's NPo does not correlate with membrane voltages (Vm). C, LTB4-induced cbv1-β1 channel activation requires optimal [Ca2+]i level. Each bar is obtained from no fewer than three membrane patches, each excised from a different oocyte. *, different from increase in NPo by 1 nm LTB4 at 10 μm [Ca2+]i (p < 0.05); #, different from increase in NPo by 1 μm LTB4 at 10 μm [Ca2+]i (p < 0.05). Error bars, S.E.
LTB4 Activation of BK Channel Required the Presence of BK β1 Subunit within the Channel Complex
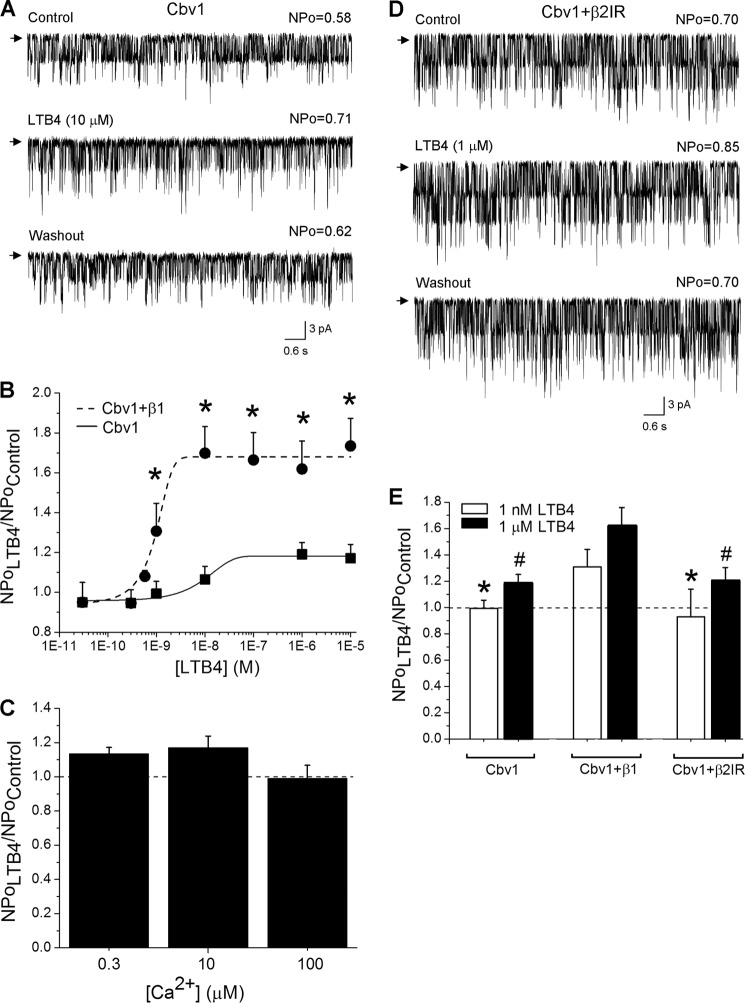
LTB4-induced activation of cbv1-β1 channels in excised patches indicated that this LTB4 action did not require the continuous presence of cytosolic factors or intracellular organelles. Therefore, it is conceivable that LTB4-induced activation of BK channels results from a direct recognition of LTB4 by the BK protein complex and/or its surrounding lipids. To determine the role of individual BK channel subunit types in LTB4 sensitivity, we tested LTB4 action on homomeric cbv1 channels heterologously expressed in Xenopus oocytes. Under experimental conditions identical to those used to study leukotriene action on cbv1-β1 complexes (see above), LTB4 caused a negligible increase in cbv1 NPo even when applied at 10 μm (Fig. 4, A and B). A lack of LTB4-induced activation of homomeric cbv1 channels was observed across a wide range of [Ca2+]i levels (Fig. 4C). Thus, our results point at the BK β1 subunit as the molecular sensor of LTB4.
FIGURE 4.
LTB4-induced BK channel activation requires the presence of BK β1 subunits. A, single-channel recordings from I/O patches excised from X. laevis oocytes expressing the cbv1 channel. Current records depict cbv1 channel activity before, during, and after 10 μm LTB4 application. Vm = −20 mV; [Ca2+]i = 10 μm. B, concentration-response curve for LTB4 in the absence of BK β1 subunit (EC50 = 80 nm). A concentration-response curve obtained from β1-containing BK channels is presented as a positive control (EC50 = 1 nm). Each point represents the average of no fewer than three patches, each patch obtained from a different oocyte. *, different from cbv1-β1 (p < 0.05). C, lack of LTB4-induced cbv1 channel activation is observed across different [Ca2+]i levels. Each bar is obtained from no fewer than three membrane patches, each excised from a different oocyte. D, single-channel recordings from I/O patches excised from X. laevis oocytes expressing the cbv1-β2IR channel. Current records depict cbv1-β2IR channel activity before, during, and after 1 μm LTB4 application. Vm = −40 mV; [Ca2+]i = 10 μm. E, averaged data showing differential effect of LTB4 on cbv1, cbv1-β1, and cbv1-β2IR channels. A horizontal dashed line highlights the level at which NPo remains unchanged. Each bar is obtained from no fewer than three membrane patches, each excised from a different oocyte. *, different from 1 nm LTB4 on cbv1-β1 (p < 0.05). #, different from 1 μm LTB4 on cbv1-β1 (p < 0.05). Error bars, S.E.
To begin to determine the specificity of LTB4 recognition by BK β1, we next tested whether β subunits other than β1 could confer LTB4 sensitivity to BK channels. Thus, we tested LTB4 on cbv1-β2IR complexes. At micromolar [Ca2+]i, β2IR-containing BK channels possess Ca2+ sensitivity similar to that of β1-containing channels (22). Remarkably, LTB4 repeatedly failed to increase cbv1-β2IR NPo to the levels obtained when the lipid was applied to cbv1-β1 (Fig. 4, D and E). Considering that 1) among β2–4 proteins, β2 has the highest identity/similarity of amino acid sequence with β1 (5), and 2) unlike β1, β2 does not confer LTB4 sensitivity to BK channel (current data), it is likely that β3 or β4 would not confer such sensitivity. Conceivably, β1 versus other β subunits may have the unique ability to enable LTB4-induced BK channel activation.
LTB4 Activates BK Channels via the Steroid-sensing Site on the BK β1 Subunit TM2
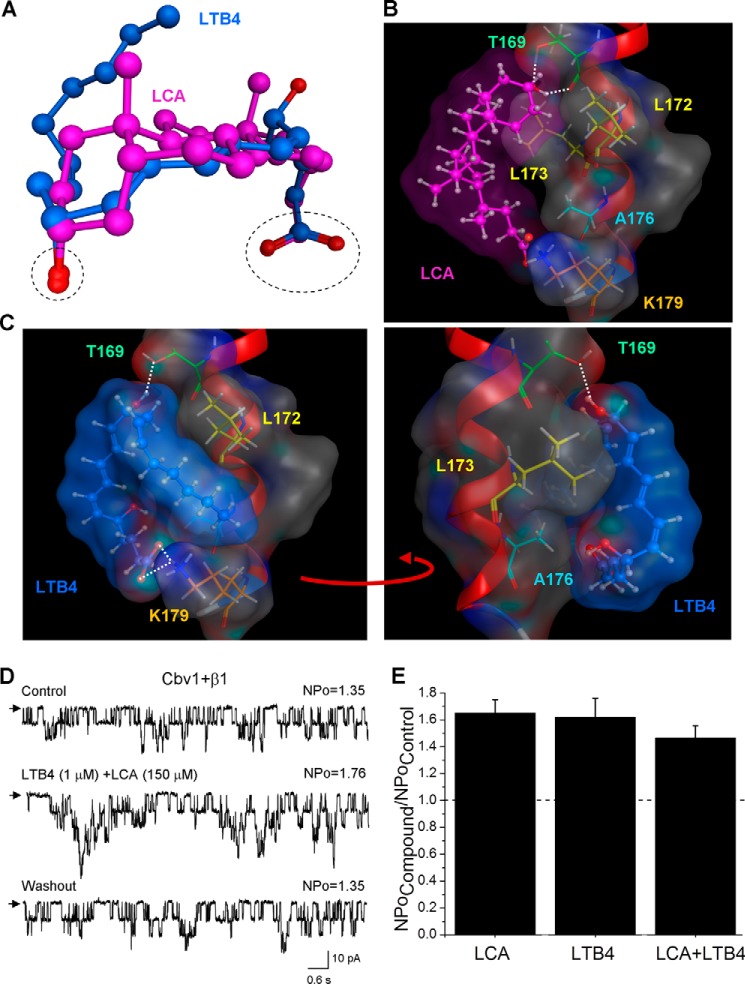
In previous work, we demonstrated that β1 subunits contain a recognition site for negatively charged cholane steroids that is not shared by β2–4 subunits. This site is located within the β1 TM2 region and requires the presence of a polar hydroxyl group within the steroid nucleus and a negatively charged carboxyl at the lateral chain of the cholane steroid molecule for ligand recognition to evoke channel activation (11, 28–29). To be an effective BK channel activator, the steroid molecule should be able to adopt a bean-like shape, which is characteristic of bile acids (11, 28). Therefore, we first compared a three-dimensional structure of LTB4 with LCA, the most effective cholane steroid activator of BK channels. Flexible superposition of these two molecules results in spatial overlap of critically important features: hydroxyl and carboxyl groups (Fig. 5A). Also, the linear but flexible LTB4 molecule is able to align along the bean-shaped LCA molecule (Fig. 5A). Therefore, from a ligand topology point of view, the cholane steroid-sensing site on the BK β1 TM2 domain represents an attractive candidate for LTB4 recognition, which results in BK channel activation.
FIGURE 5.
Computational docking of LTB4 on steroid-sensing site located on BK β1 subunit TM2. A, three-dimensional superposition of LCA (pink) and LTB4 (blue). The dashed black oval shows overlap in the spatial location of both carboxyl and oxygen groups in LCA and LTB4 molecules. The superposition shows that LTB4 can adopt the LCA-characteristic shape. B, model for LCA docking on the BK β1 subunit TM2. The steroid nucleus of LCA interacts with Thr-169, Leu-172, and Leu-173, whereas ionized carboxylate in the side chain of LCA resides near Lys-179 but does not necessarily interact with it. In B and C, the TM2 α helix is shown in red, selected β1 amino acids are shown in colors, and white dotted lines indicate hydrogen bonding. C, model for LTB4 docking on the LCA-sensing site located on BK β1 subunit TM2. The model shows tight steric interaction between LCA-sensing amino acids Thr-169, Leu-172, and Leu-173. In addition, Ala-176 and Lys-179 are proposed to play a critical role in LTB4 recognition and retention. D, single-channel recordings from I/O patches excised from X. laevis oocytes expressing the cbv1-β1 channel and probed with a mixture of 1 μm LTB4 and 150 μm LCA. Vm = −40 mV; [Ca2+]i = 10 μm. E, averaged data showing a similar increase in BK NPo by either single activator (LTB4 or LCA) or their combination. A horizontal dashed line highlights the level at which NPo remains unchanged. Each bar is obtained from no fewer than three membrane patches, each excised from a different oocyte. Error bars, S.E.
Docking of LCA onto the BK β1 steroid-sensing site involves hydrogen bonding of the LCA hydroxyl group with Thr-169 and hydrophobic interactions of the LCA steroid nucleus with Leu-172 and Leu-173 (Fig. 5B) (12). Although Ala-176 and Lys-179 are located in the vicinity of the LCA lateral chain, amino acid substitutions at these positions failed to affect LCA-induced BK channel activation (12, 28). Thus, Ala-176 and Lys-179 were ruled out as steroid-sensing amino acids.
Like LCA, docking of LTB4 onto the BK β1 TM2 domain's steroid-sensing site resulted in hydrogen bonding of one of the LTB4 hydroxyl groups with Thr-169 and placement of the LTB4 hydrocarbon chain on the hydrophobic surface formed by Leu-172 and Leu-173 (Fig. 5C, left). Unlike LCA, docking of LTB4 yielded a very tight association of the LTB4 hydrocarbon chain with the receptor surface formed by Ala-176 (Fig. 5C, right). LTB4 was also expected to form a hydrogen bond with Lys-179 (Fig. 5C, left). Therefore, in addition to previously validated steroid-sensing residues Thr-169, Leu-172, and Leu-173, the additional residues Ala-176 and Lys-179 may participate in LTB4 recognition and retention by the BK β1 TM2.
To validate our computational results, we 1) applied a mixture of LTB4 and LCA onto the cbv1-β1 channels and 2) tested LTB4 on cbv1-β1 complexes that contained substitutions of amino acids that conform the docking site. Co-application of LTB4 with LCA at concentrations that resulted in maximal BK channel activation (1 and 150 μm, respectively) failed to show additivity in increasing NPo (Fig. 5, D and E). The 1.5-fold increase in the presence of both agonists is similar to that observed in the presence of either (Fig. 5E).
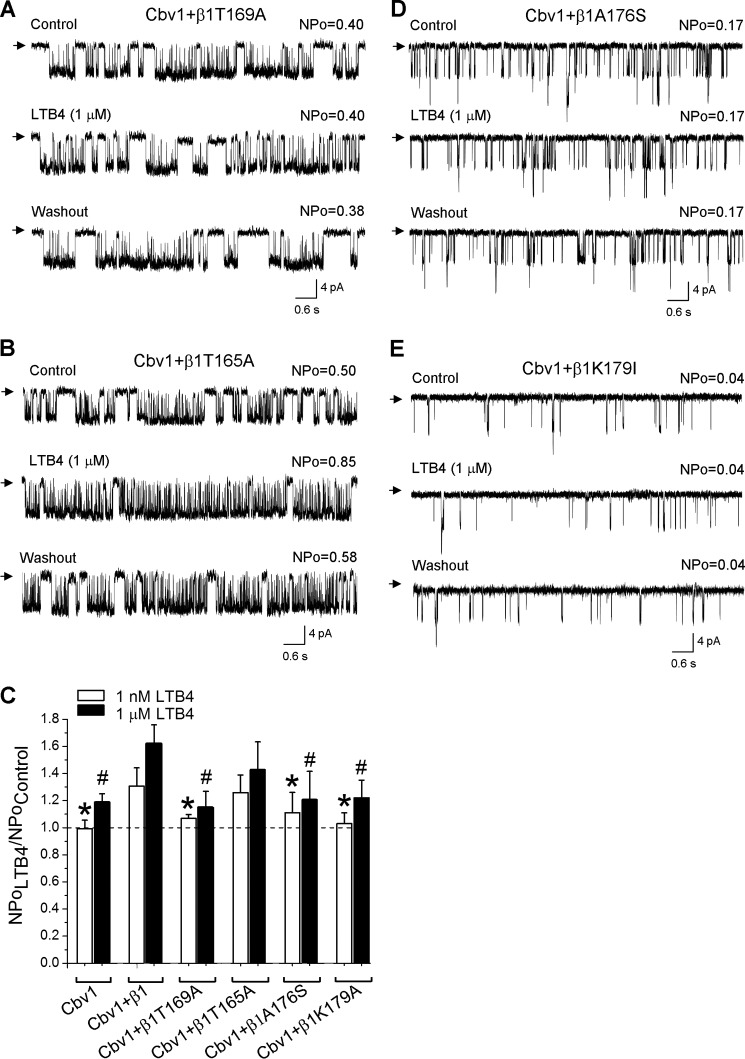
Next, we tested LTB4 on cbv1-β1 complexes with substitutions of amino acids that are predicted to form the LCA/LTB4 docking site. T169A has been proven to serve as a screening tool to identify possible ligands for the BK β1 TM2 domain steroid-sensing site (12, 30). Successful expression of β1T169A and its presence within the channel complexes were validated by detecting the β1 subunit-characteristic phenotype of cbv1-β1T169A macroscopic current (12). As expected from computational data, LTB4 repeatedly failed to increase cbv1-β1T169A NPo when tested under experimental conditions identical to those used to study leukotriene action on cbv1-β1 complexes (see above) (Fig. 6, A and C). However, LTB4-induced BK channel activation was still evident when we probed LTB4 on cbv1-β1T165A complexes, the T165A substitution targeting a threonine residue located upstream and outside the steroid-sensing area (Fig. 6, B and C). Collectively, our data underscore a distinct role for Thr-169 in LTB4 sensing by β1 subunit-containing BK channels.
FIGURE 6.
LTB4-induced BK channel activation cannot be observed in cbv1-β1 channels that contain mutations within the proposed LTB4 docking site in the BK β1 subunit. A, single-channel recordings from I/O patches excised from X. laevis oocytes expressing the cbv1-β1T169A channel. Current records depict cbv1-β1T169A channel activity before, during, and after 1 μm LTB4 application. Vm = −40 mV; [Ca2+]i = 10 μm. B, single-channel recordings from I/O patches excised from X. laevis oocytes expressing the cbv1-β1T165A channel (negative control). Current records depict cbv1-β1T165A channel activity before, during, and after 1 μm LTB4 application. Vm = −40 mV; [Ca2+]i = 10 μm. C, averaged data showing differential effect of LTB4 on cbv1, cbv1-β1, cbv1-β1T169A, cbv1-β1T165A, cbv1-β1T176S, and cbv1-β1K179A channels. A horizontal dashed line highlights the level at which NPo remains unchanged. Each bar is obtained from no fewer than three membrane patches, each excised from a different oocyte. *, different from 1 nm LTB4 on cbv1-β1 (p < 0.05); #, different from 1 μm LTB4 on cbv1-β1 (p < 0.05). D, single-channel recordings from I/O patches excised from X. laevis oocytes expressing the cbv1-β1A176S channel. Current records depict cbv1-β1A176S channel activity before, during, and after 1 μm LTB4 application. Vm = −40 mV, [Ca2+]i = 10 μm. E, single-channel recordings from I/O patches excised from X. laevis oocytes expressing the cbv1-β1K179I channel. Current records depict cbv1-β1K179I channel activity before, during, and after 1 μm LTB4 application. Vm = −40 mV; [Ca2+]i = 10 μm. Error bars, S.E.
We also tested the role of Ala-176 and Lys-179 in LTB4-sensing by testing LTB4 on cbv1-β1A176S and cbv1-β1K179I channels. The functional presence of β1A176S and β1K179I within the channel complexes was validated by the observation of the characteristic macroscopic current phenotype of β1 subunit-containing BK channels, as was done for other channel constructs (see above). LTB4 repeatedly failed to increase NPo of cbv1-β1A176S and cbv1-β1K179I complexes when tested under experimental conditions identical to those used to study leukotriene action on cbv1-β1 channels (Fig. 6, C–E). These electrophysiological data are in agreement with our computational prediction regarding a critical role of Ala-176 and Lys-179 in LTB4 sensing (Fig. 5C).
To determine whether leukotrienes that were unable to activate BK channels could also dock on the LTB4-sensing site, we tested BK channel activity in the presence of 1 μm LTB4 mixed with a 1 μm concentration of each of the non-activating leukotrienes: LTA4, LTC4, LTD4, and LTE4. In all cases, LTB4 failed to increase BK NPo in the presence of non-activating leukotrienes (Fig. 7A). One possible explanation for the loss of LTB4 activity is that the non-activating leukotrienes still dock onto the LTB4-sensing site, but this docking does not transform into modifying channel activity but rather prevents LTB4 from docking. Alternatively, non-activating leukotrienes might dock elsewhere and either prevent LTB4 docking via allosteric mechanisms or decrease channel activity, thus counterbalancing LTB4-induced BK channel activation. Our computational docking data strongly favor the first possibility; all non-activating leukotrienes were able to dock onto the LTB4-sensing site (Fig. 7B and Table 1). They form a hydrogen bond with Lys-179 and probably interact with hydrophobic amino acids within the site. In some cases, non-activating leukotrienes (LTA4 and LTC4) were unable to form a hydrogen bond with Thr-169, a key residue that is required for the LTB4-sensing site. In other cases (LTD4 and LTE4), hydrogen bond formation was quite possible, yet it would require an energetically unfavorable conformation of the LT4 molecule. In all cases, however, docking of non-activating leukotrienes on the LTB4-sensing site was expected to hinder LTB4 docking.
FIGURE 7.

Effect of LTB4 is hindered by the presence of non-activating leukotrienes of the LT4 family. A, averaged data showing the loss of LTB4-induced BK channel activation in the presence of either LTA4, LTC4, LTD4, or LTE4. A horizontal dashed line highlights the level at which NPo remains unchanged. Each bar is obtained from no fewer than three membrane patches, each excised from a different oocyte. *, different from 1 μm LTB4 on cbv1-β1 (p < 0.05). B, computational docking of non-activating leukotrienes on the LTB4-sensing site in the BK β1 subunit. Representative docking pose of each non-activating leukotriene is superposed onto the model of LTB4 docking. The LTB4 molecule in space-filling format is shown in blue; the BK β1 transmembrane helix is shown in red; non-activating leukotriene molecular structures are presented as green sticks; oxygen atoms are given in red. Hydrogen bonds are highlighted by white dotted lines. Error bars, S.E.
TABLE 1.
Characteristics of leukotriene docking on the BK channel β1 subunit
| Leukotriene | Docking on LCA site | Interaction with Lys-179 | Hydrogen bonding with Thr-169 | Steric hindrance to prevent LTB4 docking |
|---|---|---|---|---|
| LTA4 | Yes | Yes | No | Yes |
| LTB4 | Yes | Yes | Yes | |
| LTC4 | Yes | Yes | No | Yes |
| LTD4 | Yes | Yes | Slight possibility | Yes |
| LTE4 | Yes | Yes | Slight possibility | Yes |
DISCUSSION
Regulation of ion channel function, of BK in particular, due to direct interaction between ion channel protein and physiologically relevant endogenous lipids represents a growing area of research (1, 31). Indeed, several recent studies have shown direct modulation of BK channel function by fatty acids (10, 32), phosphatidylinositol 4,5-bisphosphate and other phosphoinositides (8), membrane phosphoglycerides (33), and physiologically relevant steroids, including cholesterol (7, 34), stress (35) and sex hormones (9), and bile acids and related cholane steroids (12, 28). Here, we have demonstrated for the first time that LTB4, a member of the leukotriene family of lipids, is one of the most potent endogenous lipid activators of BK channels.
Previous studies have documented a modulatory action of leukotrienes on ion channels other than BK. LTC4 produces a concentration-dependent increase in steady-state, GTPγS-mediated IKAch current of bullfrog atrial myocytes (36). LTD4, in turn, activates a chloride conductance in hepatocytes of lipopolysaccharide-treated rats (37) and both charybdotoxin-sensitive and -insensitive potassium channels in Ehrlich ascites tumor cells (38). LTB4, in particular, mediates inflammation via TRPV1 in duct obstruction-induced pancreatitis in rats (39). This leukotriene also increases RyR-mediated currents in microsomes prepared from canine cerebellum (40). In all of these studies of leukotriene-ion channel interactions, however, the mechanisms and site of leukotriene action remain undetermined. In the present work, we clearly demonstrate that BK channel modification of steady-state activity occurs at voltage and [Ca2+]i levels that are not only physiological but also optimal for BK β1 subunit control of channel function (41). Moreover, we identify for the first time this subunit as the LTB4 molecular sensor.
Remarkably, the BK β1 subunit represents a common target for several negatively charged lipids that increase BK channel activity. Indeed, the BK β1 subunit confers BK channel sensitivity to bile acids (12) and arachidonic acid (42) and significantly enhances phosphatidylinositol 4,5-bisphosphate-induced BK channel activation (8). The second transmembrane domain of the BK β1 subunit contains the cholane steroid-sensing site, which includes Thr-169, Leu-172, and Leu-173 (12). In this work, we demonstrated that this site can also accommodate LTB4. Therefore, two endogenous lipids from different chemical groups (heterocyclic cholane steroids and linear leukotrienes) can share their site of action on an ion channel protein, an unusual phenomenon because lipid-sensing sites on ion channels, BK in particular, do not share ligands from different lipid species (1, 43). The physiological implications of such overlap remain to be explored. However, our findings do not necessarily mean that the cholane steroid-sensing site could be targeted by any negatively charged lipid of physiological relevance known to regulate BK channel activity. For instance, docosahexaenoic acid activates BK currents via two amino acids located in the N terminus and first transmembrane region of the BK β1 protein (10). Although these residues may play a regulatory role rather than provide the actual docking area for docosahexaenoic acid, docosahexaenoic acid lacks a hydroxyl group that could potentially interact with Thr-169 of the cholane-sensing site. Our studies clearly indicate that this interaction is critical for lipid-induced channel activation via the steroid-sensing site on the BK β1 TM2 domain, whether involving cholane steroids (11, 12, 29), heterocyclic synthetic analogs (30), or LTB4 itself (Fig. 6). Moreover, the presence of a hydroxyl group within the hydrocarbon core of LTB4 explains the unique ability of LTB4 to activate the BK channel when compared with other members of the LT4 family. Indeed, although LTA4, LTC4, LTD4, and LTE4 are able to dock onto the LTB4-sensing site and probably prevent LTB4 from effective docking, these non-activating leukotrienes do not possess a hydroxyl or any other polar group at a position in the molecule that would favor hydrogen bonding with Thr-169 (Fig. 7).
A unique feature of LTB4-induced BK channel activation is the high potency of LTB4. Compared with other known BK channel activators (and lipid activators in particular), LTB4-induced BK channel activation could be observed at low nanomolar concentrations (EC50 ∼1 nm). Indeed, other known lipid activators of BK channel require tens of micromolar in concentration to increase channel activity (8, 28, 32, 44, 45). Our data indicate that LTB4 is one of the most potent BK channel activators ever found (Fig. 2B); its potency is matched only by that of the synthetic agent bis(1,3-dibutylbarbituric acid)trimethine oxonol and the herb-derived dehydrosoyasaponin I, with these two agents being effective at 10 nm (46, 47). The high potency of LTB4 when compared with other known BK channel activators can be explained by several factors, including unrestricted access to the transmembrane sensing site and an ability to occupy the site with high affinity. Bile acids, however, are known to cross biological membranes with ease (48), yet these cholane steroids require micromolar concentrations to evoke detectable increases in BK channel activity (11, 28).
Our present results show significant hydrophobic contacts between the LTB4 molecule and the Leu-172, Leu-173, Ala-176 hydrophobic triplet. In contrast, this type of interaction is either minimal (for Leu-172 and Leu-173) or not present (for Ala-176) when LCA docks onto the site (12). Moreover, a lack of hydrophobic interactions between LCA molecule and Leu-172, Leu-173, and Ala-176 may result in reduced binding affinity because it is generally accepted that adding a hydrophobic surface(s) to a series of analogs leads to an increase in ligand-receptor binding energy (49). Therefore, a tight fit into the cholane-sensing site may explain or at least contribute to the exceptionally high potency of LTB4 (Figs. 5 and 7) when compared with the cholane steroids.
The high potency of LTB4 is attractive for a drug development effort to design a new class of potent BK channel activators. Another advantage of using LTB4 as a template for drug design stems from the ability of LTB4 to activate BK channels when applied to the extracellular side of the membrane (Fig. 2E). In contrast, the highly potent dehydrosoyasaponin I is effective only when applied to the intracellular side of the membrane (46). This fact limits the pharmaceutical potential of dehydrosoyasaponin I because dehydrosoyasaponin I-derived drugs would be ineffective when administered via conventional routes (e.g. intravenous delivery or oral administration). Finally, LTB-induced BK channel activation is favored by physiologically relevant levels of [Ca2+]i (Fig. 3, B and C). This characteristic is also important for developing new therapeutic compounds.
In conclusion, we identified for the first time LTB4 as a novel activator of BK channels, such interaction requiring direct recognition by the channel regulatory β1 subunit, and documented the ability of endogenously produced lipids from different chemical groups to share a common sensing site on the ion channel accessory subunit.
Acknowledgment
We deeply thank Maria Asuncion-Chin (University of Tennessee Health Science Center) for excellent technical assistance.
This work was supported, in whole or in part, by National Institutes of Health Grants AA020184 (to A. B.) and HL10463-01 and AA11560 (to A. D.).
- BK
- large conductance, calcium- and voltage-gated potassium
- [Ca2+]i
- calcium concentration at the intracellular membrane leaflet
- HEDTA
- N-(2-hydroxyethyl)ethylenediamine-N,N′,N′-triacetic acid
- I/O
- inside-out
- LCA
- lithocholic acid
- LT4
- leukotriene 4
- LTA4
- leukotriene A4
- LTB4
- leukotriene B4
- LTC4
- leukotriene C4
- LTD4
- leukotriene D4
- LTE4
- leukotriene E4
- O/O
- outside-out
- N
- number of channels in the membrane patch
- Po
- probability of channel opening
- TM
- transmembrane protein region
- Vm
- voltage difference across the membrane
- GTPγS
- guanosine 5′-3-O-(thio)triphosphate.
REFERENCES
- 1. Poveda J. A., Giudici A. M., Renart M. L., Molina M. L., Montoya E., Fernández-Carvajal A., Fernández-Ballester G., Encinar J. A., González-Ros J. M. (2014) Lipid modulation of ion channels through specific binding sites. Biochim. Biophys. Acta 1838, 1560–1567 [DOI] [PubMed] [Google Scholar]
- 2. Orio P., Rojas P., Ferreira G., Latorre R. (2002) New disguises for an old channel: MaxiK channel β-subunits. News Physiol. Sci. 17, 156–161 [DOI] [PubMed] [Google Scholar]
- 3. Weaver A. K., Liu X., Sontheimer H. Role for calcium-activated potassium channels (BK) in growth control of human malignant glioma cells. J. Neurosci. Res. 78, 224–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Essin K., Gollasch M., Rolle S., Weissgerber P., Sausbier M., Bohn E., Autenrieth I. B., Ruth P., Luft F. C., Nauseef W. M., Kettritz R. (2009) BK channels in innate immune functions of neutrophils and macrophages. Blood 113, 1326–1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brenner R., Jegla T. J., Wickenden A., Liu Y., Aldrich R. W. (2000) Cloning and functional characterization of novel large conductance calcium-activated potassium channel β subunits, hKCNMB3 and hKCNMB4. J. Biol. Chem. 275, 6453–6461 [DOI] [PubMed] [Google Scholar]
- 6. Behrens R., Nolting A., Reimann F., Schwarz M., Waldschütz R., Pongs O. (2000) hKCNMB3 and hKCNMB4, cloning and characterization of two members of the large-conductance calcium-activated potassium channel β subunit family. FEBS Lett. 474, 99–106 [DOI] [PubMed] [Google Scholar]
- 7. Singh A. K., McMillan J., Bukiya A. N., Burton B., Parrill A. L., Dopico A. M. (2012) Multiple cholesterol recognition/interaction amino acid consensus (CRAC) motifs in cytosolic C tail of Slo1 subunit determine cholesterol sensitivity of Ca2+- and voltage-gated K+ (BK) channels. J. Biol. Chem. 287, 20509–20521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vaithianathan T., Bukiya A., Liu J., Liu P., Asuncion-Chin M., Fan Z., Dopico A. (2008) Direct regulation of BK channels by phosphatidylinositol 4,5-bisphosphate as a novel signaling pathway. J. Gen. Physiol. 132, 13–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Valverde M. A., Rojas P., Amigo J., Cosmelli D., Orio P., Bahamonde M. I., Mann G. E., Vergara C., Latorre R. (1999) Acute activation of Maxi-K channels (hSlo) by estradiol binding to the β subunit. Science 285, 1929–1931 [DOI] [PubMed] [Google Scholar]
- 10. Hoshi T., Tian Y., Xu R., Heinemann S. H., Hou S. (2013) Mechanism of the modulation of BK potassium channel complexes with different auxiliary subunit compositions by the ω-3 fatty acid DHA. Proc. Natl. Acad. Sci. U.S.A. 110, 4822–4827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bukiya A. N., McMillan J., Parrill A. L., Dopico A. M. (2008) Structural determinants of monohydroxylated bile acids to activate beta 1 subunit-containing BK channels. J. Lipid Res. 49, 2441–2451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bukiya A. N., Singh A. K., Parrill A. L., Dopico A. M. (2011) The steroid interaction site in transmembrane domain 2 of the large conductance, voltage- and calcium-gated potassium (BK) channel accessory β1 subunit. Proc. Natl. Acad. Sci. U.S.A. 108, 20207–20212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Peters-Golden M., Henderson W. R., Jr. (2007) Leukotrienes. N. Engl. J. Med. 357, 1841–1854 [DOI] [PubMed] [Google Scholar]
- 14. Shiratsuki N., Uyama O., Kitada O., Suenaga N., Nakamura H., Sugita M., Hayashi Y., Yamamoto S. (1990) Effects of hydrocortisone and aminophylline on plasma leukotriene C4 levels in patients during an asthmatic attack. Prostaglandins Leukot. Essent. Fatty Acids 40, 285–289 [DOI] [PubMed] [Google Scholar]
- 15. Shindo K., Miyakawa K., Fukumura M. (1993) Plasma levels of leukotriene B4 in asthmatic patients. Int. J. Tissue React. 15, 181–184 [PubMed] [Google Scholar]
- 16. Sasagawa M., Satoh T., Takemoto A., Hasegawa T., Suzuki E., Arakawa M. (1994) Blood levels of leukotrienes (LTC4, D4, E4, B4) in asthmatic patients during attack and remission. Arerugi 43, 28–36 [PubMed] [Google Scholar]
- 17. Jaggar J. H., Li A., Parfenova H., Liu J., Umstot E. S., Dopico A. M., Leffler C. W. (2005) Heme is a carbon monoxide receptor for large-conductance Ca2+-activated K+ channels. Circ. Res. 97, 805–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bukiya A. N., Liu J., Dopico A. M. (2009) The BK channel accessory β1 subunit determines alcohol-induced cerebrovascular constriction. FEBS Lett. 583, 2779–2784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu J., Vaithianathan T., Manivannan K., Parrill A., Dopico A. M. (2008) Ethanol modulates BKCa channels by acting as an adjuvant of calcium. Mol. Pharmacol. 74, 628–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bukiya A. N., Vaithianathan T., Kuntamallappanavar G., Asuncion-Chin M., Dopico A. M. (2011) Smooth muscle cholesterol enables BK β1 subunit-mediated channel inhibition and subsequent vasoconstriction evoked by alcohol. Arterioscler. Thromb. Vasc. Biol. 31, 2410–2423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang Y. W., Ding J. P., Xia X. M., Lingle C. J. (2002) Consequences of the stoichiometry of Slo1 α and auxiliary β subunits on functional properties of large-conductance Ca2+-activated K+ channels. J. Neurosci. 22, 1550–1561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Orio P., Latorre R. (2005) Differential effects of β1 and β2 subunits on BK channel activity. J. Gen. Physiol. 125, 395–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Knot H. J., Nelson M. T. (1998) Regulation of arterial diameter and wall [Ca2+] in cerebral arteries of rat by membrane potential and intravascular pressure. J. Physiol. 508, 199–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pérez G. J., Bonev A. D., Nelson M. T. (2001) Micromolar Ca2+ from sparks activates Ca2+-sensitive K+ channels in rat cerebral artery smooth muscle. Am. J. Physiol. Cell. Physiol. 281, C1769–C1775 [DOI] [PubMed] [Google Scholar]
- 25. Tanaka Y., Koike K., Alioua A., Shigenobu K., Stefani E., Toro L. (2004) β1-subunit of MaxiK channel in smooth muscle: a key molecule which tunes muscle mechanical activity. J. Pharmacol. Sci. 94, 339–347 [DOI] [PubMed] [Google Scholar]
- 26. Cox D. H., Aldrich R. W. (2000) Role of the β1 subunit in large-conductance Ca2+-activated K+ channel gating energetics. Mechanisms of enhanced Ca2+ sensitivity. J. Gen. Physiol. 116, 411–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Meera P., Wallner M., Jiang Z., Toro L. (1996) A calcium switch for the functional coupling between α (hslo) and β subunits (KV,Ca β) of maxi K channels. FEBS Lett. 382, 84–88 [DOI] [PubMed] [Google Scholar]
- 28. Dopico A. M., Walsh J. V., Jr., Singer J. J. (2002) Natural bile acids and synthetic analogues modulate large conductance Ca2+-activated K+ (BKCa) channel activity in smooth muscle cells. J. Gen. Physiol. 119, 251–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bukiya A. N., Vaithianathan T., Toro L., Dopico A. M. (2008) The second transmembrane domain of the large conductance, voltage- and calcium-gated potassium channel β(1) subunit is a lithocholate sensor. FEBS Lett. 582, 673–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bukiya A. N., McMillan J. E., Fedinec A. L., Patil S. A., Miller D. D., Leffler C. W., Parrill A. L., Dopico A. M. (2013) Cerebrovascular dilation via selective targeting of the cholane steroid-recognition site in the BK channel β1-subunit by a novel nonsteroidal agent. Mol. Pharmacol. 83, 1030–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hou S., Heinemann S. H., Hoshi T. (2009) Modulation of BKCa channel gating by endogenous signaling molecules. Physiology 24, 26–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Clarke A. L., Petrou S., Walsh J. V., Jr., Singer J. J. (2002) Modulation of BK(Ca) channel activity by fatty acids: structural requirements and mechanism of action. Am. J. Physiol. Cell. Physiol. 283, C1441–C1453 [DOI] [PubMed] [Google Scholar]
- 33. Crowley J. J., Treistman S. N., Dopico A. M. (2005) Distinct structural features of phospholipids differentially determine ethanol sensitivity and basal function of BK channels. Mol. Pharmacol. 68, 4–10 [DOI] [PubMed] [Google Scholar]
- 34. Bukiya A. N., Belani J. D., Rychnovsky S., Dopico A. M. (2011) Specificity of cholesterol and analogs to modulate BK channels points to direct sterol-channel protein interactions. J. Gen. Physiol. 137, 93–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. King J. T., Lovell P. V., Rishniw M., Kotlikoff M. I., Zeeman M. L., McCobb D. P. (2006) β2 and β4 subunits of BK channels confer differential sensitivity to acute modulation by steroid hormones. J. Neurophysiol. 95, 2878–2888 [DOI] [PubMed] [Google Scholar]
- 36. Scherer R. W., Lo C. F., Breitwieser G. E. (1993) Leukotriene C4 modulation of muscarinic K+ current activation in bullfrog atrial myocytes. J. Gen. Physiol. 102, 125–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Meng X. J., Carruth M. W., Weinman S. A. (1997) Leukotriene D4 activates a chloride conductance in hepatocytes from lipopolysaccharide-treated rats. J. Clin. Invest. 99, 2915–2922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hoffmann E. K. (1999) Leukotriene D4 (LTD4) activates charybdotoxin-sensitive and -insensitive K+ channels in Ehrlich ascites tumor cells. Pflugers Arch. 438, 263–268 [DOI] [PubMed] [Google Scholar]
- 39. Vigna S. R., Shahid R. A., Nathan J. D., McVey D. C., Liddle R. A. (2011) Leukotriene B4 mediates inflammation via TRPV1 in duct obstruction-induced pancreatitis in rats. Pancreas 40, 708–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Striggow F., Ehrlich B. E. (1997) Regulation of intracellular calcium release channel function by arachidonic acid and leukotriene B4. Biochem. Biophys. Res. Commun. 237, 413–418 [DOI] [PubMed] [Google Scholar]
- 41. Bao L., Cox D. H. (2005) Gating and ionic currents reveal how the BKCa channel's Ca2+ sensitivity is enhanced by its β1 subunit. J. Gen. Physiol. 126, 393–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Martín P., Moncada M., Enrique N., Asuaje A., Valdez Capuccino J. M., Gonzalez C., Milesi V. (2014) Arachidonic acid activation of BKCa (Slo1) channels associated to the β1-subunit in human vascular smooth muscle cells. Pflugers Arch. 466, 1779–1792 [DOI] [PubMed] [Google Scholar]
- 43. Dopico A. M., Bukiya A. N. (2014) Lipid regulation of BK channel function. Front. Physiol. 5, 312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kirber M. T., Ordway R. W., Clapp L. H., Walsh J. V., Jr., Singer J. J. (1992) Both membrane stretch and fatty acids directly activate large conductance Ca2+-activated K+ channels in vascular smooth muscle cells. FEBS Lett. 297, 24–28 [DOI] [PubMed] [Google Scholar]
- 45. Bukiya A. N., Liu J., Toro L., Dopico A. M. (2007) β1 (KCNMB1) subunits mediate lithocholate activation of large-conductance Ca2+-activated K+ channels and dilation in small, resistance-size arteries. Mol. Pharmacol. 72, 359–369 [DOI] [PubMed] [Google Scholar]
- 46. McManus O. B., Harris G. H., Giangiacomo K. M., Feigenbaum P., Reuben J. P., Addy M. E., Burka J. F., Kaczorowski G. J., Garcia M. L. (1993) An activator of calcium-dependent potassium channels isolated from a medicinal herb. Biochemistry. 32, 6128–6133 [DOI] [PubMed] [Google Scholar]
- 47. Morimoto T., Sakamoto K., Sade H., Ohya S., Muraki K., Imaizumi Y. (2007) Voltage-sensitive oxonol dyes are novel large-conductance Ca2+-activated K+ channel activators selective for β1 and β4 but not for β2 subunits. Mol. Pharmacol. 71, 1075–1088 [DOI] [PubMed] [Google Scholar]
- 48. Cabral D. J., Small D. M., Lilly H. S., Hamilton J. A. (1987) Transbilayer movement of bile acids in model membranes. Biochemistry 26, 1801–1804 [DOI] [PubMed] [Google Scholar]
- 49. Snyder P. W., Mecinovic J., Moustakas D. T., Thomas S. W., 3rd, Harder M., Mack E. T., Lockett M. R., Héroux A., Sherman W., Whitesides G. M. (2011) Mechanism of the hydrophobic effect in the biomolecular recognition of arylsulfonamides by carbonic anhydrase. Proc. Natl. Acad. Sci. U.S.A. 108, 17889–17894 [DOI] [PMC free article] [PubMed] [Google Scholar]