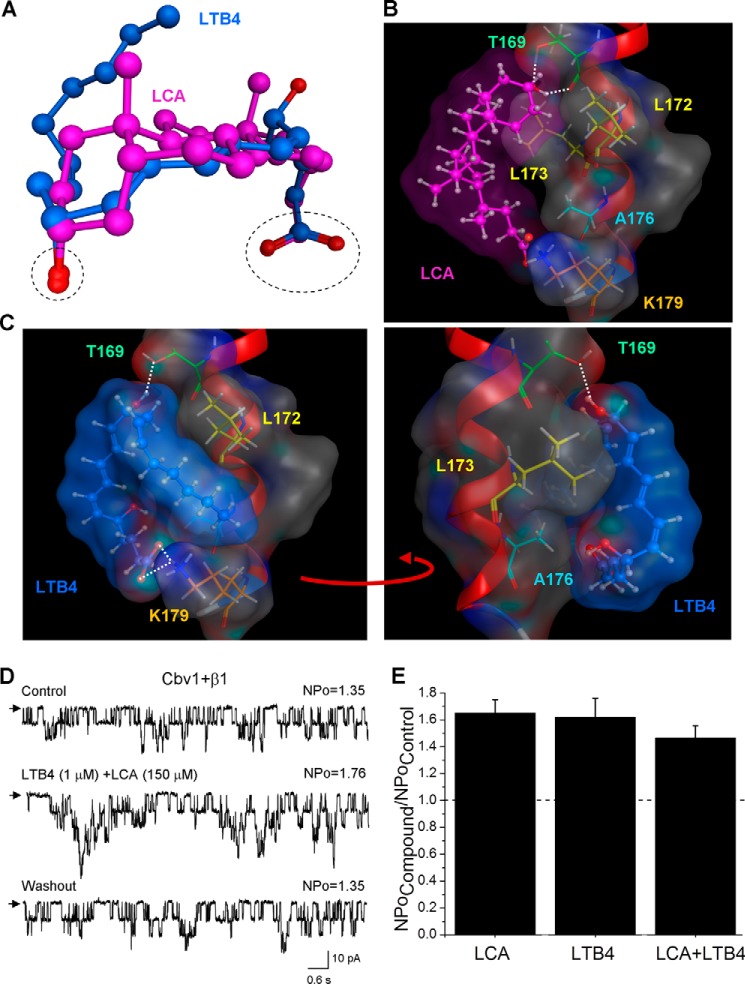
FIGURE 5.
Computational docking of LTB4 on steroid-sensing site located on BK β1 subunit TM2. A, three-dimensional superposition of LCA (pink) and LTB4 (blue). The dashed black oval shows overlap in the spatial location of both carboxyl and oxygen groups in LCA and LTB4 molecules. The superposition shows that LTB4 can adopt the LCA-characteristic shape. B, model for LCA docking on the BK β1 subunit TM2. The steroid nucleus of LCA interacts with Thr-169, Leu-172, and Leu-173, whereas ionized carboxylate in the side chain of LCA resides near Lys-179 but does not necessarily interact with it. In B and C, the TM2 α helix is shown in red, selected β1 amino acids are shown in colors, and white dotted lines indicate hydrogen bonding. C, model for LTB4 docking on the LCA-sensing site located on BK β1 subunit TM2. The model shows tight steric interaction between LCA-sensing amino acids Thr-169, Leu-172, and Leu-173. In addition, Ala-176 and Lys-179 are proposed to play a critical role in LTB4 recognition and retention. D, single-channel recordings from I/O patches excised from X. laevis oocytes expressing the cbv1-β1 channel and probed with a mixture of 1 μm LTB4 and 150 μm LCA. Vm = −40 mV; [Ca2+]i = 10 μm. E, averaged data showing a similar increase in BK NPo by either single activator (LTB4 or LCA) or their combination. A horizontal dashed line highlights the level at which NPo remains unchanged. Each bar is obtained from no fewer than three membrane patches, each excised from a different oocyte. Error bars, S.E.