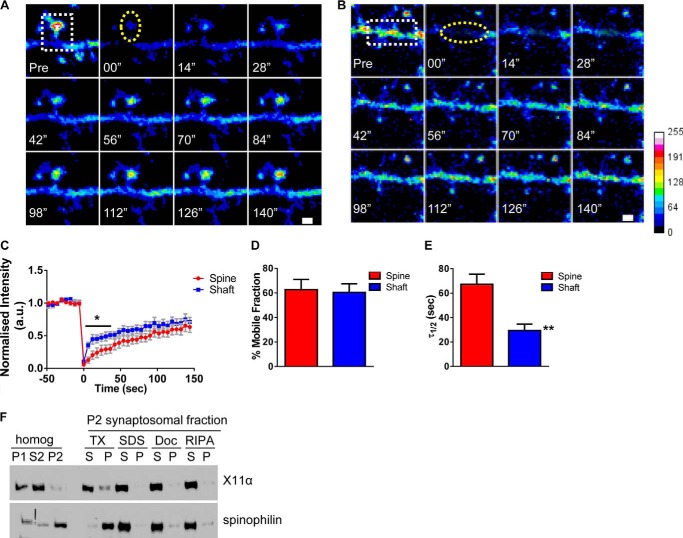
FIGURE 4.
X11α is a mobile protein in dendrites and synapses. A and B, time-lapse FRAP imaging of GFP-X11α in dendritic spine (A) and in dendrites (B). Square regions encompassing spines or dendritic shaft were photobleached (white dotted square) using 10 passes (12 s total) of 100% laser power, which was optimized to quench fluorescence in fixed cells. Recovery fluorescence was acquired using 1% laser power, with images taken every 6 s for 144 s. Intensity of recovered fluorescence was measured in spine region or dendritic shaft region (yellow dashed ovals). Focal drift was adjusted for by measuring intensity of nonbleached area on a different dendrite of the same cell. C, normalized intensity of GFP-X11α signal during fluorescence recovery after photobleaching. D, percentage of GFP-X11α that is mobile in spines and dendritic shaft, as measured by percentage of fluorescence that recovers after photobleaching. E, τ½ (time at which 50% of equilibrium fluorescence level is recovered) for regions of interest in spines and dendritic shafts. τ½ of GFP-X11α in dendritic shaft is significantly shorter than dendritic spines. F, relative abundance of X11α and spinophilin in rat cortex PSD fractions. Synaptosomes were prepared from rat cortical tissue using a HEPES/sucrose gradient, separated by SDS-PAGE, and analyzed by Western blotting using X11α (upper) and spinophilin (lower) antibodies. Fractions indicated are as follows: P1, nuclear pellet; S2, supernatant from crude synaptosomal fraction; P2, crude synaptosomal pellet. The P2 fraction was further solubilized in buffers with detergents of different strengths, and soluble (S) or insoluble and particulate (P) fractions were separated by centrifugation. Detergents used were as follows: TX, Triton X-100; SDS, sodium dodecyl sulfate; Doc, deoxycholate. X11α was present in P1, S2, and P2 fractions and was extracted from synaptosome fractions by all detergents tested, suggesting a weaker association with the PSD. **, p < 0.001. Scale bar, 1 μm.