FIGURE 5.
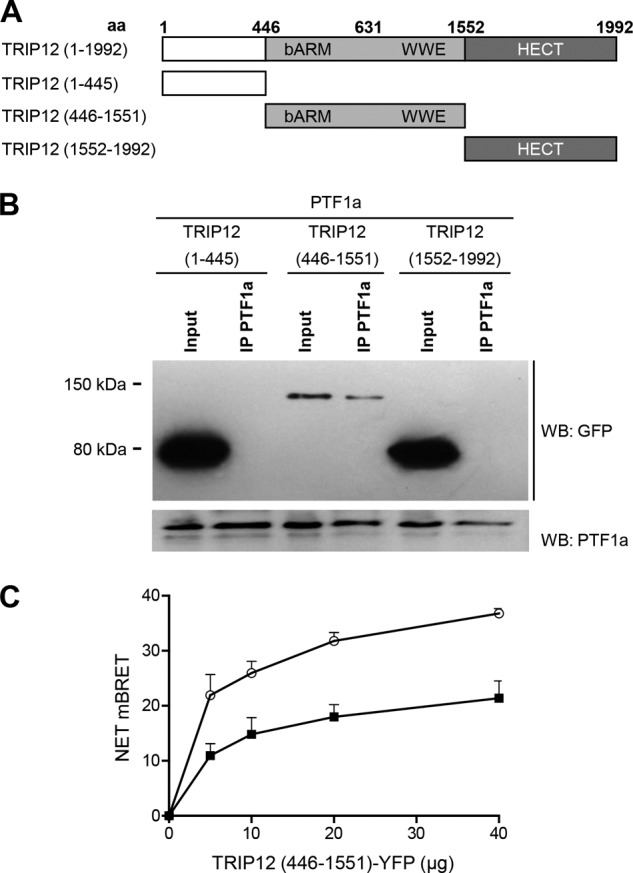
The N-terminal region and the HECT domain of TRIP12 are not involved in PTF1a recognition. A, wild-type and truncated TRIP12 proteins. aa, amino acid. All constructs were GFP-tagged. B, HEK-293T cells were co-transfected with PTF1a and vectors for GFP-tagged truncated TRIP12 proteins as indicated. Cell lysates were immunoprecipitated or not (Input) with an anti-PTF1a antibody then analyzed by Western blot using anti-GFP or anti-PTF1a antibodies. A negative control consisting of anti-rabbit IgG beads alone led to no band (not shown). C, HEK-293T cells were transfected separately with the BRET donor (C- or N-terminally labeled PTF1a-HRluc vector) or the BRET acceptor (C-terminal labeled TRIP12 (446–1551) mutant-YFP vector). After 48 h, total cellular proteins were extracted. A total of 3 μg of lysate containing the BRET donor was mixed with variable amounts (0–40 μg) of lysate containing the BRET acceptor. White circles, C-terminal labeled PTF1a-HRluc vector; black squares, N-terminal labeled PTF1a-HRluc vector. The Rluc substrate coelenterazin-h was added 15 min before reading. The data represent the net BRET values expressed in NET milli BRET units. WB, Western blot.
