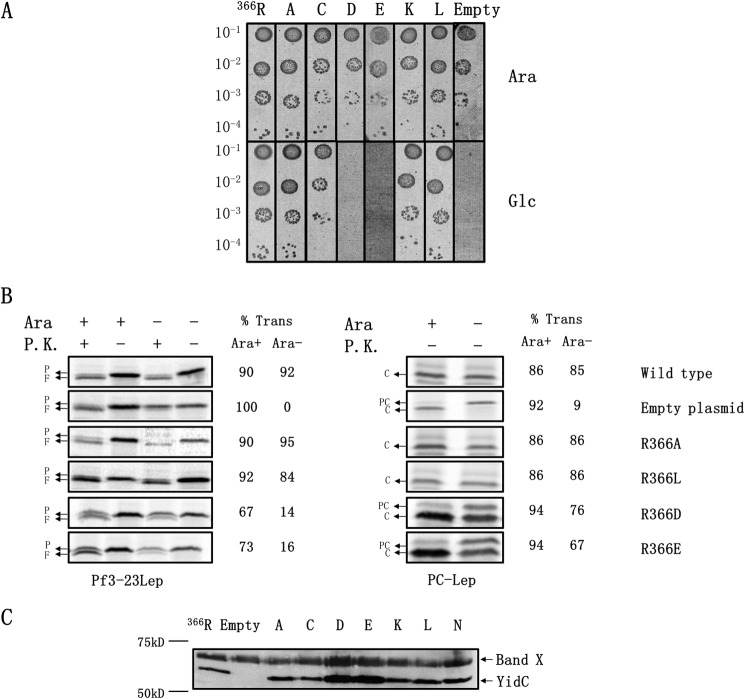
FIGURE 2.
Arginine 366 in the E. coli YidC is dispensable for function. A, complementation assay to test the importance of Arg-366 for E. coli YidC function. Arg-366 within the E. coli YidC was mutated to Ala, Cys, Asp, Glu, Lys, and Leu in pACYC184. The pACYC184-encoded YidC mutants were transformed and expressed in E. coli JS7131 under YidC depletion conditions for 3 h. Expression of the plasmid-encoded YidC mutants was under the endogenous yidC promoter. Serial dilutions of the cultures were spotted and incubated under wild-type YidC expression conditions (top panels) and depletion conditions (lower panels), respectively. Plasmid-encoded wild-type YidC was also expressed, as a positive control, to show it was capable of supporting growth of the cell when chromosomal YidC was depleted. B, E. coli YidC neutral and negatively charged mutants at 366 were tested for their ability to promote the insertion of Pf3-23Lep and PC-Lep. JS7131 encoding the pACYC184-YidC wild-type, mutants, or the pACYC184 empty vector were grown for 3 h under YidC expression (0.2% arabinose) or YidC depletion conditions (0.2% glucose). The respective strains were co-transformed with pMS119 encoding Pf3-23Lep or PC-Lep. Expression of the YidC substrate was induced by the addition of 1 mm isopropyl β-d-1-thiogalactopyranoside, and the cells were labeled with [35S]methionine for 1 min. Protease accessibility assay (left panels) was used to monitor translocation of the N-tail of Pf3-23Lep. After [35S] labeling, the cells were converted to spheroplasts, and a portion was treated with proteinase K (P.K.), as described under “Experimental Procedures.” Signal peptide processing (right panels) was employed to measure membrane insertion of PC-Lep. After labeling with [35S]methionine for 1 min, the cells were precipitated with TCA and analyzed as described under “Experimental Procedures.” The percent translocation of Pf3-23Lep and PC-Lep was quantified as described under “Experimental Procedures.” P represents Pf3-23Lep protein; F denotes the proteinase K fragment; PC represents PC-Lep; C denotes C-Lep protein generated by SP1 cleavage. C, Western blot to examine expression and stability of the E. coli YidC mutants using antiserum against a C-terminal peptide of EcYidC. The top band is an unrelated 70-kDa protein recognized by our antiserum. P.K., proteinase K.