Figure 1.
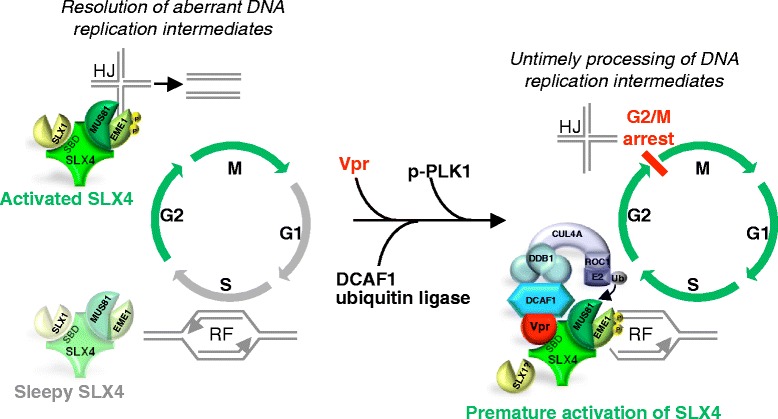
Model for Vpr-mediated G2 arrest through remodeling of the SLX4 complex. In a physiological contex activation of the SLX4 complex (SLX4com) intervenes to allow the resolution of DNA replication intermediates, such as Holliday Junctions (HJ), found in collapsed replication forks after DNA replication (phases of SLX4 activation are symbolized by green arrows). This leads to a proper G2/M phase transition (left panel). HIV-1 Vpr protein interacts with SLX4 on its SLX1 binding domain, the CUL4A–DDB1DCAF1 ubiquitin ligase and PLK1/p-PLK1. These interactions lead to MUS81 ubiquitination, and phosphorylation of EME1 by p-PLK1, resulting in aberrant activation of SLX4com. This inappropriate activation perturbs progression of ongoing replication forks (RF) in S phase and the resolution of abnormal DNA replication intermediates. As a consequence, cells arrest at the G2 phase of the cell cycle (right panel).
