Abstract
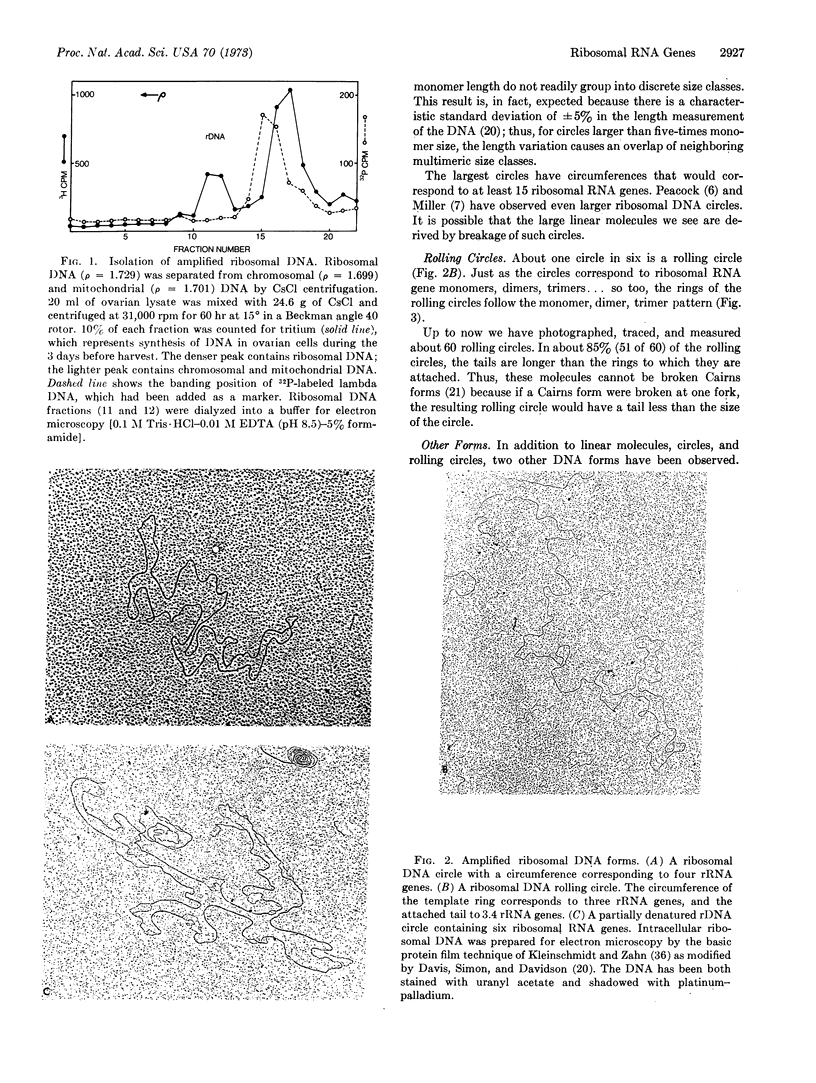
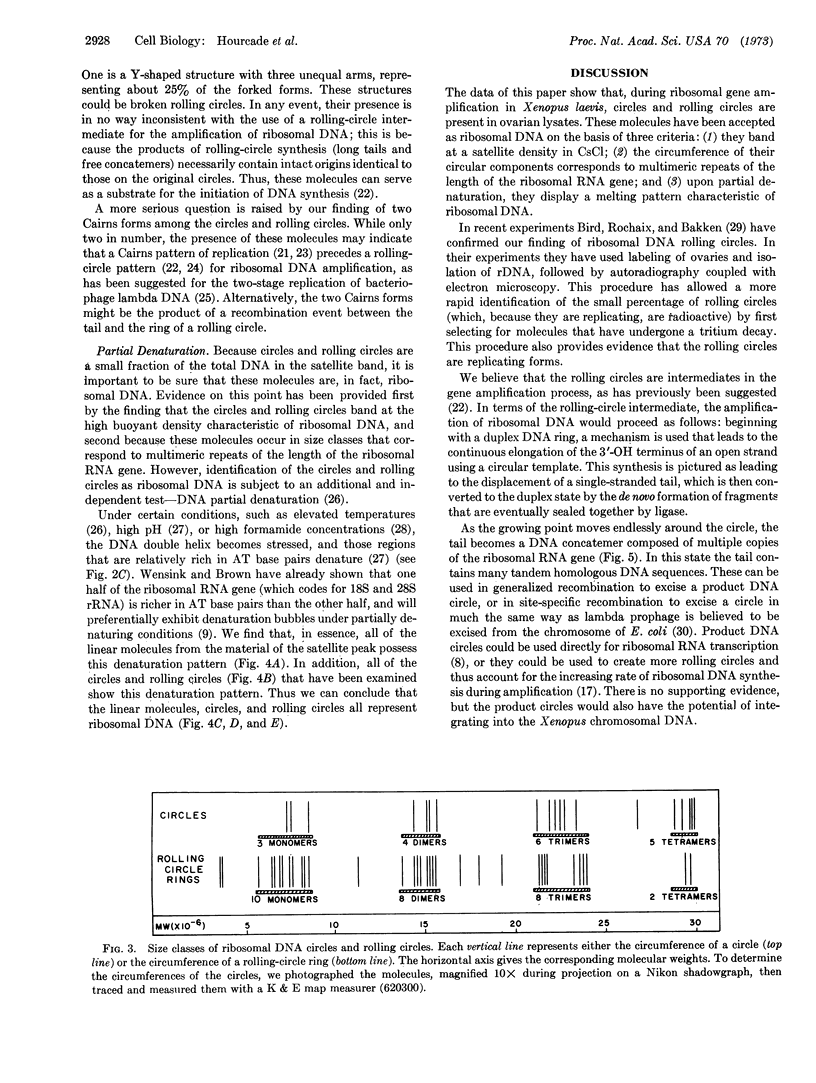
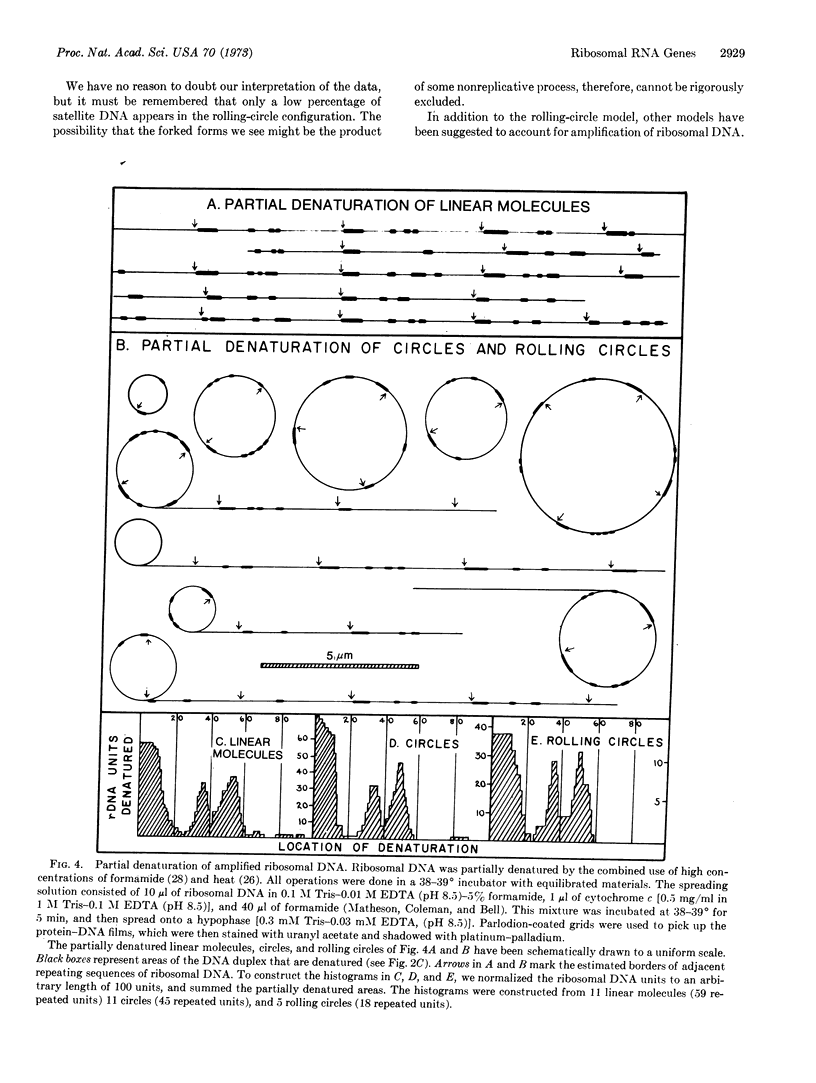
During the development of Xenopus oocytes there is a special DNA synthesis that leads to a thousandfold amplification of the genes that code for ribosomal RNA. We have used the electron microscope to study this process. Our primary observation is the presence of ribosomal DNA in rolling-circle intermediates at the time of amplification. We believe that these intermediates are involved in the amplification process, and as such offer the first example of the involvement of a rolling circle in the replication of eukaryotic DNA.
Keywords: DNA replication, Xenopus laevis, gene amplification, repetitive DNA, electron microscopy of DNA
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltimore D. RNA-dependent DNA polymerase in virions of RNA tumour viruses. Nature. 1970 Jun 27;226(5252):1209–1211. doi: 10.1038/2261209a0. [DOI] [PubMed] [Google Scholar]
- Bird A., Rogers E., Birnstiel M. Is gene amplification RNA-directed? Nat New Biol. 1973 Apr 25;242(121):226–230. doi: 10.1038/newbio242226a0. [DOI] [PubMed] [Google Scholar]
- Birnstiel M., Speirs J., Purdom I., Jones K., Loening U. E. Properties and composition of the isolated ribosomal DNA satellite of Xenopus laevis. Nature. 1968 Aug 3;219(5153):454–463. doi: 10.1038/219454a0. [DOI] [PubMed] [Google Scholar]
- Brown D. D., Dawid I. B. Specific gene amplification in oocytes. Oocyte nuclei contain extrachromosomal replicas of the genes for ribosomal RNA. Science. 1968 Apr 19;160(3825):272–280. doi: 10.1126/science.160.3825.272. [DOI] [PubMed] [Google Scholar]
- Brown D. D., Weber C. S. Gene linkage by RNA-DNA hybridization. II. Arrangement of the redundant gene sequences for 28 s and 18 s ribosomal RNA. J Mol Biol. 1968 Jun 28;34(3):681–697. doi: 10.1016/0022-2836(68)90189-7. [DOI] [PubMed] [Google Scholar]
- Brown D. D., Wensink P. C., Jordan E. A comparison of the ribosomal DNA's of Xenopus laevis and Xenopus mulleri: the evolution of tandem genes. J Mol Biol. 1972 Jan 14;63(1):57–73. doi: 10.1016/0022-2836(72)90521-9. [DOI] [PubMed] [Google Scholar]
- Buongiorno-Nardelli M., Amaldi F., Lava-Sanchez P. A. Amplification as a rectification mechanism for the redundant rRNA genes. Nat New Biol. 1972 Aug 2;238(83):134–137. doi: 10.1038/newbio238134a0. [DOI] [PubMed] [Google Scholar]
- Carter B. J., Shaw B. D., Smith M. G. Two stages in the replication of bacteriophage lambda DNA. Biochim Biophys Acta. 1969 Dec 16;195(2):494–505. doi: 10.1016/0005-2787(69)90656-x. [DOI] [PubMed] [Google Scholar]
- Crippa M., Tocchini-Valentini G. P. Synthesis of amplified DNA that codes for ribosomal RNA. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2769–2773. doi: 10.1073/pnas.68.11.2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawid I. B., Brown D. D., Reeder R. H. Composition and structure of chromosomal and amplified ribosomal DNA's of Xenopus laevis. J Mol Biol. 1970 Jul 28;51(2):341–360. doi: 10.1016/0022-2836(70)90147-6. [DOI] [PubMed] [Google Scholar]
- Eisen H., Pereira da Silva L., Jacob F. The regulation and mechanism of DNA synthesis in bacteriophage lambda. Cold Spring Harb Symp Quant Biol. 1968;33:755–764. doi: 10.1101/sqb.1968.033.01.086. [DOI] [PubMed] [Google Scholar]
- Gall J. G. Differential synthesis of the genes for ribosomal RNA during amphibian oögenesis. Proc Natl Acad Sci U S A. 1968 Jun;60(2):553–560. doi: 10.1073/pnas.60.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall J. G. The genes for ribosomal RNA during oögenesis. Genetics. 1969;61(1 Suppl):121–132. [PubMed] [Google Scholar]
- Gilbert W., Dressler D. DNA replication: the rolling circle model. Cold Spring Harb Symp Quant Biol. 1968;33:473–484. doi: 10.1101/sqb.1968.033.01.055. [DOI] [PubMed] [Google Scholar]
- Inman R. B. Denaturation maps of the left and right sides of the lambda DNA molecule determined by electron microscopy. J Mol Biol. 1967 Aug 28;28(1):103–116. doi: 10.1016/s0022-2836(67)80081-0. [DOI] [PubMed] [Google Scholar]
- Inman R. B., Schnös M. Partial denaturation of thymine- and 5-bromouracil-containing lambda DNA in alkali. J Mol Biol. 1970 Apr 14;49(1):93–98. doi: 10.1016/0022-2836(70)90378-5. [DOI] [PubMed] [Google Scholar]
- Landesman R., Gross P. R. Patterns of macromolecule synthesis during development of Xenopus laevis. II. Identification of the 40 S precursor to ribosomal RNA. Dev Biol. 1969 Mar;19(3):244–260. doi: 10.1016/0012-1606(69)90063-3. [DOI] [PubMed] [Google Scholar]
- Miller O. L., Jr, Beatty B. R. Extrachromosomal nucleolar genes in amphibian oocytes. Genetics. 1969;61(1 Suppl):133–143. [PubMed] [Google Scholar]
- Miller O. L., Jr Structure and composition of peripheral nucleoli of salamander oocytes. Natl Cancer Inst Monogr. 1966 Dec;23:53–66. [PubMed] [Google Scholar]
- Painter T. S., Taylor A. N. Nucleic Acid Storage in the Toad's Egg. Proc Natl Acad Sci U S A. 1942 Aug;28(8):311–317. doi: 10.1073/pnas.28.8.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peacock W. J. Chromosome replication. Natl Cancer Inst Monogr. 1965 Dec;18:101–131. [PubMed] [Google Scholar]
- Sebring E. D., Kelly T. J., Jr, Thoren M. M., Salzman N. P. Structure of replicating simian virus 40 deoxyribonucleic acid molecules. J Virol. 1971 Oct;8(4):478–490. doi: 10.1128/jvi.8.4.478-490.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temin H. M., Mizutani S. RNA-dependent DNA polymerase in virions of Rous sarcoma virus. Nature. 1970 Jun 27;226(5252):1211–1213. doi: 10.1038/2261211a0. [DOI] [PubMed] [Google Scholar]
- Vinograd J., Lebowitz J., Radloff R., Watson R., Laipis P. The twisted circular form of polyoma viral DNA. Proc Natl Acad Sci U S A. 1965 May;53(5):1104–1111. doi: 10.1073/pnas.53.5.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace H., Birnstiel M. L. Ribosomal cistrons and the nucleolar organizer. Biochim Biophys Acta. 1966 Feb 21;114(2):296–310. doi: 10.1016/0005-2787(66)90311-x. [DOI] [PubMed] [Google Scholar]
- Wensink P. C., Brown D. D. Denaturation map of the ribosomal DNA of Xenopus laevis. J Mol Biol. 1971 Sep 14;60(2):235–247. doi: 10.1016/0022-2836(71)90290-7. [DOI] [PubMed] [Google Scholar]
- Wolfson J., Dressler D., Magazin M. Bacteriophage T7 DNA replication: a linear replicating intermediate (gradient centrifugation-electron microscopy-E. coli-DNA partial denaturation). Proc Natl Acad Sci U S A. 1972 Feb;69(2):499–504. doi: 10.1073/pnas.69.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]





