Figure 2.
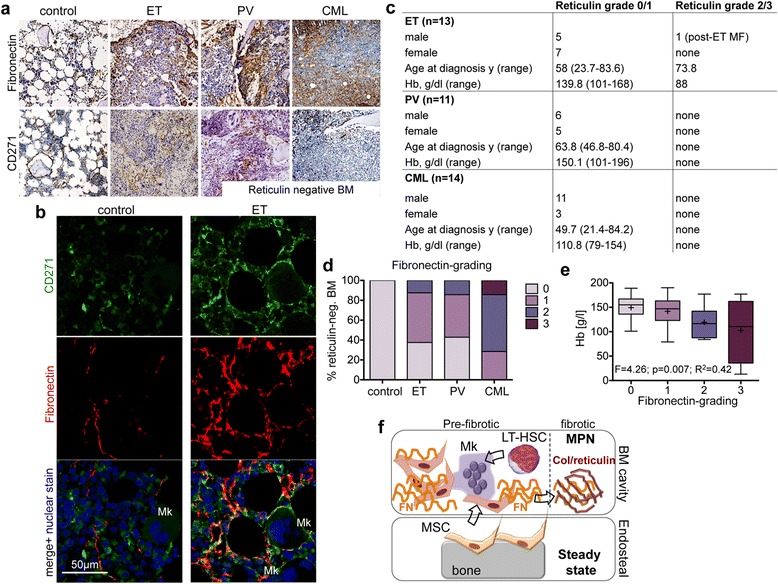
In vivo validation of the fibronectin-secretory phenotype of MPN-MSC. (a) Representative Fibronectin and CD271 expression in correlating bone marrow biopsies of MPN-MSC donors. (b) Co-localization of fibronectin and CD271 expression by confocal microscopy. CD271 (green, Alexa Fluor 488) and fibronectin (red, Alexa Fluor 555) were analysed separately (panel 1 and 2) and merged (panel 3) with the nuclear counterstain TO-PRO®-3 Iodide (blue, excitation at 633 nm). Cytoplasmatic extensions of CD271+ MSC co-localize with Fibronectin, in particular in association to megakaryocytes (Mk). (c) Clinical and laboratory characteristics of patient samples included in the tissue microarray (TMA). y, years; Hb, haemoglobin levels. (d) Fibronectin grading was adapted to Reticulin grading (Thiele et al.) in reticulin-negative MPN TMA samples. (e) Correlation of fibronectin-grading in reticulin-negative graded TMA samples to haemoglobin levels. Box plots show minimal, quartile, median and maximal values in each group, (+) = mean value. (f) Schematic depicting the hypothesis that MSC reside in the endosteal (and vascular) niche under steady state conditions and are activated from these niche by dysplastic megakaryocytes (Mk) and the neoplastic clone (LT-HSC). Once activated, MPN-MSC acquire a secretory phenotype that facilitates MSC migration, matrix contraction and provides a provisional matrix for collagen (Col) fibres in more progressed fibrosis.
