Figure 3.
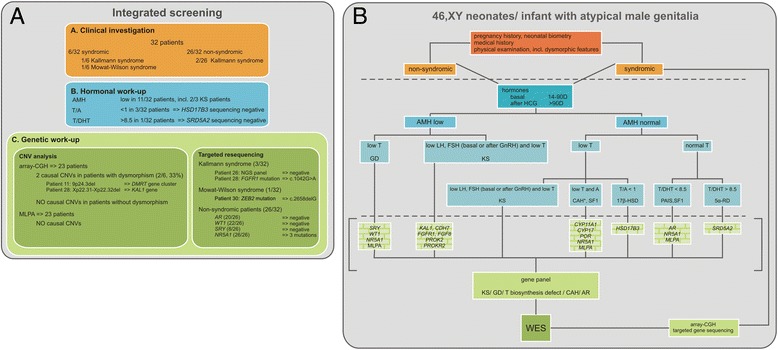
Overview of the integrated investigation approach. (A) Results in the 46,XY undervirilization cohort. Clinical and hormonal investigation was sufficient to suspect a diagnosis in 4/32 cases. For two Kallmann syndrome patients the diagnosis was genetically confirmed, as shown in the CNV analysis and targeted resequencing boxes. A ZEB2 mutation was identified in the Mowat-Wilson syndrome patient. Subsequently a genetic work-up was performed for the remaining patients, guided by hormonal results. Sequencing of HSD17B3 and SRD5A2 in patients with a possible testosterone biosynthesis disorder did not reveal mutations. Genetic screening consisting of array-CGH, DSD MLPA and sequential gene-by-gene sequencing led to the identification of two causal CNVs (of which one KS, see above) and three novel NR5A1 mutations, respectively. (B) Suggested clinical algorithm for the investigation of 46,XY male neonates or infants referred for atypical genitalia. Upper section (orange): clinical investigation, including pregnancy history, medical history and physical examination, enables categorization in cases with and without syndromic features. . Mid-section (blue): In all cases, clinical investigation should be followed by a hormonal work-up, which in turn can be suggestive of gonadal dysgenesis (GD), disorders of the steroid hormone biosynthesis pathway and/or rare forms of CAH (*:Only forms characterized by defective androgen production are implicated here), partial androgen receptor defects or KS. Insights in hormone levels can guide selection of target candidate genes. Lower section (green): After thorough evaluation of clinical and hormonal data, a decision can be made to sequence specific gene panels or to proceed to clinical whole exome sequencing to identify the underlying molecular cause and thereby support the clinical diagnosis. The boxes between brackets (with squared filling) represent single gene tests which can be replaced be the aforementioned gene panels In cases with syndromic features, array-CGH is still a recommended method to identify CNVs.
