Abstract
Polycyclic aromatic hydrocarbons (PAHs) and arsenic are both environmental agents that are known to have significant immunotoxicity. Previous studies have shown that PAH exposure of spleen cells in vitro produces significant immune suppression of humoral immunity, especially when P450 activation products are examined. Exposure to arsenic, particularly sodium arsenite, has also been found to be suppressive to antibody responses in vitro and in vivo. The purpose of the present studies was to examine the immunotoxicity of PAHs and arsenite following co-exposures with the theory being that the agents may exert synergistic actions which might be based on their different mechanisms of action. Spleen cells were isolated from male C57BL/6J wild-type mice and treated with PAHs and/or arsenic (arsenite or arsenate). Immunotoxicity assays were used to assess the T-dependent antibody response (TDAR) to sheep red blood cells (SRBC), measured by a direct plaque forming cell (PFC) assay. Cell viability was measured by trypan blue staining. Spleen cell viability was not altered following four days of PAH and/or arsenic treatment. However, the TDAR response demonstrated suppression by both PAHs or arsenic in a concentration-dependent manner. p53 was also induced by NaAsO2 (As+3) and PAHs alone or in combination. The PAHs and their metabolites investigated included benzo[a]pyrene (BaP), BaP-7,8-diol, BaP-7,8-diol-9,10-epoxide (BPDE), 7,12-dimethylbenz[a]anthracene (DMBA), DMBA-3,4-diol, dibenzo[a,l]pyrene (DB[a,l]P). PAH metabolites were found to be more potent than parent compounds in producing immunosuppression and inducing p53 expression. Interestingly, DB[a,l]P, a potent carcinogenic PAH not previously characterized for immunotoxicity, was also found to be strongly immunosuppressive. Arsenite (NaAsO2, As+3) was found to produce immunosuppression at concentrations as low as 0.5 µM and was immunosuppressive at a 10-fold lower concentration than sodium arsenate (Na2HAsO4, As+5). Co-exposure of spleen cell cultures to PAHs and As+3, both at individual low-effect concentrations, was found to produce profound suppression of the TDAR demonstrating synergy between these two chemical classes of agents.
Keywords: immunotoxicity, PAHs, arsenic, synergy, TDAR
Introduction
Polycyclic aromatic hydrocarbons (PAHs) such as benzo[a]pyrene (BaP) and inorganic arsenic are toxicologically important compounds that are widely distributed in the environment. Environmentally, these agents co-exist at sites of fossil fuel combustion, in cigarette smoke, migration from hazardous waste sites, and in drinking water (Li et al., 2009). Humans are exposed daily to mixtures of these components through ingestion and inhalation, and children may be especially sensitive to these combined exposures (Roberts et al., 2009). In addition, occupational exposures to arsenic in nonferrous smelters, pesticide manufacturing, or from consumption of contaminated drinking water, coupled with tobacco use, represents another important source for exposure to PAHs and arsenic mixtures.
PAHs are well-characterized xenobiotics that suppress a variety of innate and adaptive responses in mice and human peripheral blood mononuclear cells (Davila et al., 1996). The immunosuppressive effects of PAHs have been well-characterized by our laboratory using several immune function assays; however, the most sensitive assay has proved to be the primary antibody response to sheep red blood cells (SRBC). This T cell -dependent antibody response (TDAR) assay is recommended by the Environmental Protection Agency (EPA) for evaluation of new chemicals to be used in the environment (White et al., 2010).
7,12-dimethylbenz[a]anthracene (DMBA) has been used as a model PAH for evaluation of immunotoxicity in our laboratory. Previous studies have shown that DMBA and its metabolites persistently suppress both humoral and cell-mediated immune responses in various species both in vitro and in vivo (Burchiel et al., 1990; Burchiel et al., 1992; Gao et al., 2005). p53, cytochromeP450 1B1 (CYP1B1) and microsomal epoxide hydrolase (mEH, EPHX1 gene) are all required for the spleen cell immunosuppression produced by DMBA in vivo, demonstrating that metabolic activation and genotoxicity is important in PAH-mediated immunosuppression (Gao et al., 2005; Gao et al., 2007a,b). There are, however, important differences between DMBA and other PAHs, such as BaP, in the amount of immunosuppression that is produced in vivo in mice. BaP has been found to be significantly less immunotoxic in vivo than DMBA, perhaps due to the observation that BaP is mostly metabolized in the liver to non-immunotoxic metabolites by AhR-induced CYP1A1 and CYP1A2 (Uno et al., 2004, 2006; Dong et al., 2009). Conversely, we have found that DMBA is a poor AhR-dependent inducer of CYP1A1 and in fact produces its immunotoxicity via mostly AhR-independent mechanisms involving CYP1B1 in target tissues (Gao et al., 2005).
Inorganic arsenicals, including arsenite (As+3) and arsenate (As+5) are relatively ubiquitous in the environment. Chronic arsenic toxicity in humans has been documented in many countries worldwide, particularly in countries of Southeast Asia. In humans, arsenic compounds undergo reduction, methylation, and glutathione conjugation to yield polar metabolites that are substrates for transporters followed by excretion in the urine (Carter et al., 2003). Inorganic arsenic has been demonstrated to interact with PAHs and UVA in carcinogenesis (Maier et al., 2002; Rossman, 2003; Evans et al., 2004). Previous studies have shown that arsenite co-treatment enhances the formation of stable BaP–DNA adducts and this increase is cytochrome CYP1A1-dependent (Maier et al., 2002; Evans et al., 2004). Immunotoxic effects of arsenic have been demonstrated in various animal models (Snow et al., 2005; Cohen et al., 2006; Burchiel et al., 2009). Inhalation exposure of arsenic trioxide in C57BL/6J mice inhibited the TDAR response (Burchiel et al., 2009). B6C3F1 mice exposed to a single dose of gallium arsenide (GaAs) exhibited suppressed T cell proliferation and macrophage activity, along with a reduction in IgM and IgG production (Burns et al., 1991). Also, the immunization of GaAs-treated mice with sheep red blood cells produced a major decrease in CD4+ spleen cells (Sikorski et al., 1989). Children exposed to arsenic in drinking water have been reported to have disorders in several lymphocyte subpopulations and altered cytokine secretion (Soto-Peña et al., 2006).
Based on the fact that PAHs and arsenic are each known to produce immunosuppression and these chemical classes appear to interact in carcinogenesis bioasaays, the present study was designed to determine if the combination of these agents increases the risk for immunotoxicity in C57BL/6J mice spleen cells. In vitro exposures were used in order to minimize the pharmacokinetic effect of in vivo metabolic transformation and systematic organ distribution in mice, as well as to assess the direct immunotoxic effects of xenobiotics on spleen cells. Our results demonstrate that low concentrations of sodium arsenite potentiate the immunotoxicity of low concentrations of PAHs and their metabolites. In addition, we show that both As+3 and PAHs induce p53 in murine spleen cells in vitro, suggesting a possible mechanism whereby these agents may interact.
Materials and Methods
Chemicals and reagents
Sodium arsenite (NaAsO2, As+3), sodium arsenate (Na2HAsO4, As+5), benzo[a]pyrene (BaP), 7,12-dimethylbenz[a]anthracene (DMBA) and dibenzo[a,l]pyrene (DB [a,l]P) were purchased from Sigma-Aldrich (St. Louis, MO). BaP-trans-7,8-dihydrodiol (BP-diol), BaP-trans-7,8-dihydrodiol-9,10-epoxide (BPDE) and DMBA-trans-3,4-dihydrodiol (DMBA-diol) were obtained from National Cancer Institute Chemical Repository (Midwest Research Institute, Kansas City, MO). Sodium arsenite/arsenate were dissolved in tissue culture grade water, PAHs were dissolved in tissue culture grade anhydrous dimethylsulfoxide (DMSO; Sigma, St. Louis, MO). Both water and DMSO served as the solvent controls. The final concentrations of water and DMSO in all cell cultures were 0.1%. Solvents at these concentrations were found to be without measurable effect on the TDAR response. Cell culture materials were from Sigma-Aldrich and Invitrogen (Grand Island, NY).
Animals
Male C57BL/6J mice (6–8 weeks old) were purchased from Jackson Laboratories (Bar Harbor, ME) and were housed in our Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC)-accredited animal facility under an Institutional Animal Care and Use Committee-approved protocol. In all of the experiments, mice were euthanized by CO2, followed by spleen cell isolation, described below. All mice were used at the age of 10–14 weeks. Mice spleen weights were recorded at the time of euthanasia.
Spleen cell preparation
Single cell suspensions were prepared and combined from three individual mice for each treatment group. Spleen cells were harvested as described previously (Gao at al., 2005). In brief, spleens were isolated in RPMI 1640 complete medium supplemented with 10% fetal bovine serum, 2 mM L-glutamine, 100 µg/ml streptomycin and 100 Units/ml penicillin, and centrifuged at 280 × g for 10 min. Cell pellets were resuspended and maintained in RPMI 1640 complete medium on ice. 3 ml of media was used for each spleen. Viable spleen cell counts were obtained using the trypan blue (Sigma Chemical Co., St. Louis, MO) exclusion method and counted with a hemacytometer. Under the conditions used in our studies none of the PAHs or arsenicals produced significant cytotoxicity.
In vitro treatment and plaque-forming cell assay
Mouse spleen cells collected sterilely (4 × 106 cells/ml, 0.5 ml) were treated with arsenic and/or PAHs and cultured for four days with 0.5 ml of washed 1% sheep red blood cells (SRBC) (Colorado Serum, Denver, CO) in 48-well, flat-bottomed plates (Corning Glass, Corning, NY) with RPMI 1640 medium [containing 10% heat inactive fetal bovine serum (Atlanta Biologicals, Lawrenceville, GA), 50 µM 2-mercaptoethenol (GIBCO, Grand Island, NY), 1 mM sodium pyruvate (GIBCO, Grand Island, NY) and 50 µg/ml gentamycin (GIBCO, Grand Island, NY)]. The plates were placed in a humidified incubator at 37°C in a 5% CO2 atmosphere. RPMI 1640 medium without SRBC was added to the spleen cells as a control using a modified Mishell and Dutton (1967) approach. Quadruplicate cultures were run for each treatment with SRBC. Meanwhile a control plate for checking cell viability was also set up. Four days later, a glass slide modification of Jerne and Nordin (1963) PFC assay was performed. Briefly, the immunized spleen cells were collected from individual cultures, and washed twice with RPMI 1640. The immunized spleen cells with 50 µl 67% SRBC were then added into the appropriate glass tubes. These tubes were placed in a 43°C constant temperature water bath with 400 µl 0.8% Seaplaque agarose (Intermountain Scientific, Kaysville, UT). SRBC were added to the tubes and one slide was used for each culture (quadruplicate) to determine the PFC response. The mixture of spleen cells and SRBC was poured onto 3×1×1 mm, 0.15% Seaplaque agarose precoated microscope slide and allowed to cool. The slides were incubated for 1.5 h at 37°C in a humidified in cubator. Guinea pig complement (Colorado Serum, Denver, CO) diluted in Dulbecco’s phosphate buffered saline (DPBS) with calcium + magnesium was used to flood the slides on each tray. Following an additional 2 h incubation at 37°C, the numbers of anti-SRBC plaque-forming cells (PFC) per culture were identified. The data are presented as the number of PFC/culture based on the number of total cells plated on Day 0 (2 × 106 cells per culture).
In vitro treatment of spleen cells and whole cell lysate preparation for p53 analysis
Mouse spleen cells were collected sterilely as described above (6 × 106 cells/ml, 1ml) in RPMI 1640 medium [containing 10% heat inactivated fetal bovine serum (Atlanta Biologicals, Lawrenceville, GA), 2mM L-glutamine (GIBCO, Grand Island, NY), 100 U/ml penicillin with 100 µg streptomycin sulfate (Lonza, Walkersville, MD) and 50 µg/ml gentamycin (GIBCO, Grand Island, NY)] in 48-well, flat-bottomed plates. Cells were treated with As+3 and/or PAHs and were cultured for 4 h, 8h and 18h in a humidified, 37°C, 5% CO2 incubator. To prepare the whole lysate, spleen cells from each treatment were collected in a 15 ml centrifuge tube, centrifuged at 300 × g for 10 min at 4°C. The supernatant was discarded, and the pellets were resuspended in 2 ml ammonium chloride lysing solution (10× = 1.5 M ammonium chloride, 100 mM sodium carbonate, 10 mM disodium EDTA, and water at pH 7.4). Samples were held at room temperature for 10 min, followed by washing twice with DPBS. The pellets were resuspended in 200 µl RIPA buffer (containing 50mM Tris, 150 mM sodium chloride, 0.1% SDS, 0.5% sodium deoxycholate, 1% Triton X100, 1 mM phenylmethylsulfonyl fluoride (PMSF), 1 mM sodium orthovanadate and Roche Complete Protease Inhibitor Tablet, pH 7.4). Suspensions were held on ice for 10 min, sonicated on ice for 10 sec, centrifuged at 17,900 × g for 10 min at 4°C. The supernatants were collected in a 0.65 ml microcentrifuge tubes. Total protein concentrations were determined using Protein Assay reagent (Bio-Rad Laboratories, Hercules, CA)
Western blot analysis of p53
Total p53 and beta-actin expression were analyzed as previously described (Gao et al., 2005). Briefly, 100 µg of whole cell lysate was heated at 95°C for 5 min with 6 × sample buffer (containing 0.35M Tris, pH 6.8, 30% Glycerol, 10% SDS, 0.6M dithiothreitol (DTT), 0.012% bromphenol blue). Samples were separated by SDS polyacrylamide gel electrophoresis using a 10% resolving gel and 5% stacking gel by a mini-PROTEIN 3 cell system (Bio-Rad Laboratories). After 1 h electrophoresis at 180V, proteins were transferred to nitrocellulose membranes (0.45 µm; Bio-Rad Laboratories) for 1 hr using a constant 300 mA current. Nonspecific binding was blocked by incubating membranes in 5% (w/v) Nonfat Dry Milk (Bio-Rad Laboratories) in Tris-buffered saline containing Tween [TBS/T; 50 mM Tris, pH 7.4, 150 mM NaCl, and 0.1% (v/v) Tween 20] at room temperature for 1 h. Incubation was followed by three 5 min TBS/T washes; membranes were then incubated with a p53 1C12 antibody (1:1000; Cell Signaling Technology Inc., Danvers, MA) at 4°C overnight. After washing with TBS/T, membranes were incubated with a horseradish peroxidase (HRP)-conjugated anti-mouse IgG secondary antibody (1:2000; Cell Signaling Technology Inc.) for 1 h at room temperature in TBS/T with 5% Bovine Serum Albumin (BSA, Sigma-Aldrich). The protein bands were detected using SuperSignal® West Femto Maximum Sensitivity Substrate (Thermo Scientific, Rockford, IL) and visualized on a Kodak Image Station 4000 mm (Eastman Kodak, Rochester, NY). The protein molecular weight was determined by comparison with Precision Plus Protein Western C standards (Bio-Rad Laboratories). After detection of p53 1C12 antibody, the membranes were stripped using Gentle ReView Buffer (Amresco Inc., Solon, Ohio) in a 37°C water bath for 30 min and were then reprobed with beta-actin antibody (1:500; Santa Cruz Biotechnology, Santa Cruz, CA).
Statistical analysis
All of the data reported in this paper were analyzed by SigmaStat software (Systat Software, Inc). The statistical differences were determined by a one-way analysis of variance (ANOVA). A p-value of <0.05 was considered significant. Data were reported as the average ± SEM for replicate cultures (as indicated) which measures the inter-culture variability of treatments rather than the inter-animal variation in responses.
Results
Immunosuppressive Effects of BaP, BaP-diol and BPDE on the Spleen Cell TDAR Response in C57BL/6J mice
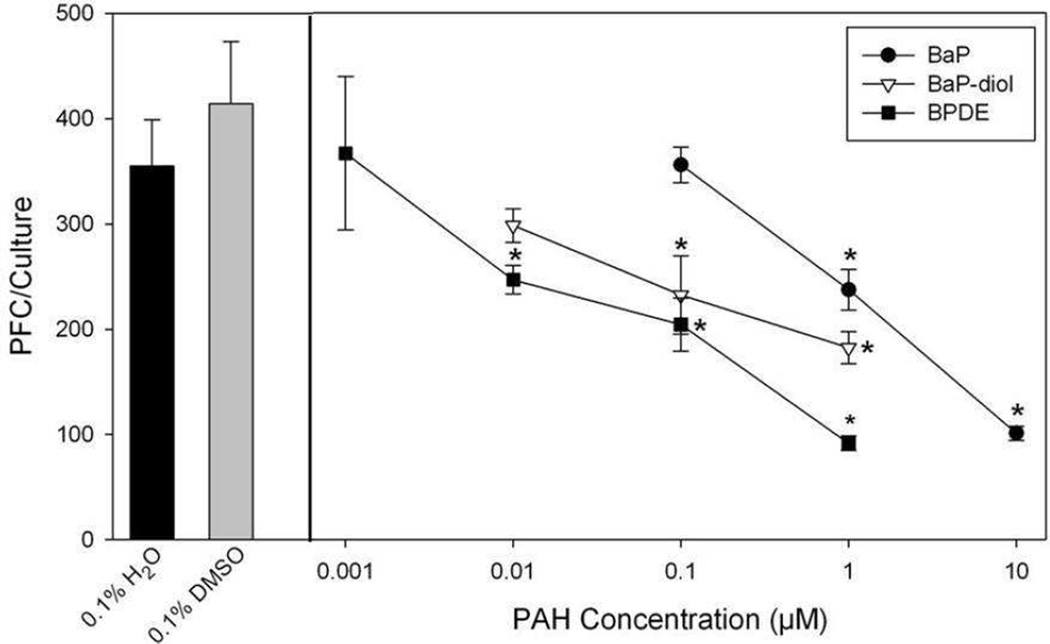
Because BaP is an important environmental PAH that is bioactivated by cells to form BP-diol, and BPDE, we compared the concentration-dependent effects of these three agents in vitro (Figure 1). These PAHs exhibited different potencies for suppression of the TDAR. At a concentration of 1 µM, BaP produced about 50% suppression of the TDAR, whereas BaP-diol was 10-fold more immunosuppressive than BaP, and BPDE was approximately 100 times more suppressive than BaP. These observations agree with our current understanding of BaP metabolism by CYPs (1A1 and 1B1) and mEH to exert its immunotoxicity.
Fig. 1.
Suppression of the TDAR by BaP, BaP-diol and BPDE in male C57BL/6J mice examined in vitro. Spleen cells were immunized with SRBC in vitro at the same time of treatment with DMSO, BaP, BaP-diol, or BPDE. The number of antibody producing spleen cells was determined by a modified Jerne and Nordin PFC assay as described in Materials and Methods. No cytotoxicity was observed in these cultures. Data from a pool of three mouse spleens that were assayed in quadruplicate are shown as mean ± S.E.M. Statistically significant differences compared with 0.1% DMSO are indicated (*, p< 0.05).
DMBA, DMBA-diol, and DB[a,l]P Suppress the Spleen Cell TDAR Response in C57BL/6J mice
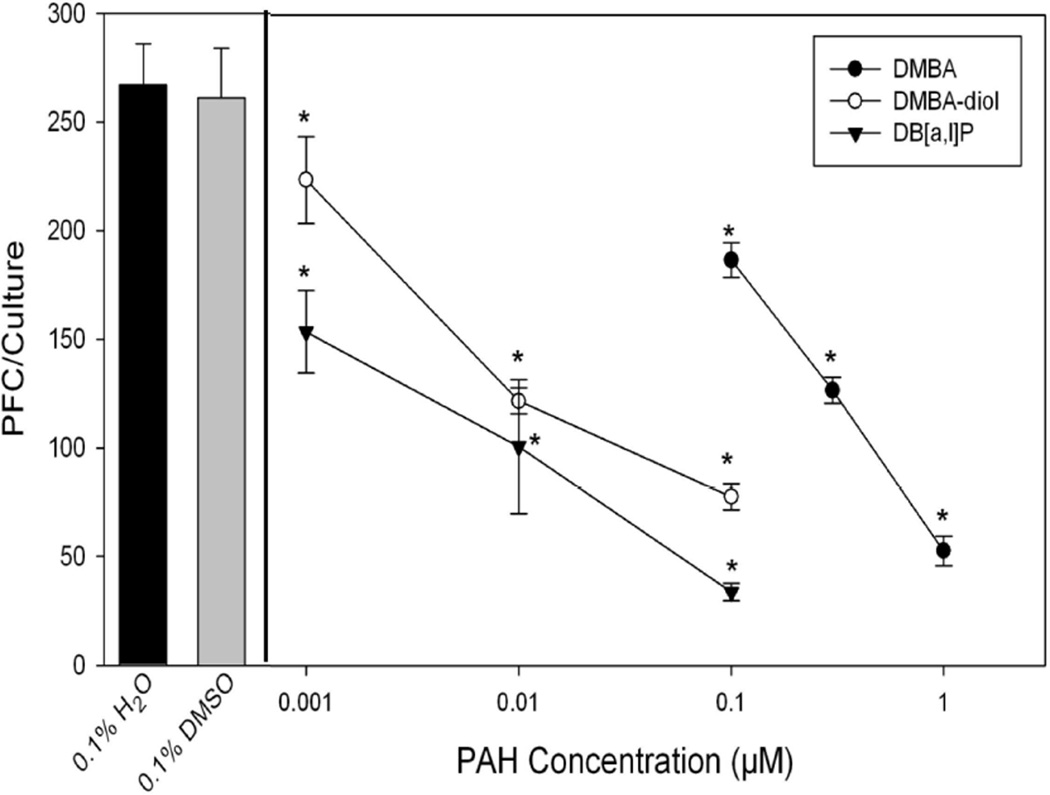
To characterize the spleen cell humoral immune response following DMBA, DMBA-diol, or DB[a,l]P treatments, the TDAR response to SRBCs was examined (Figure 2). DMBA-3,4-diol is a metabolically CYP-activated form of DMBA (Lau et al., 1995). DB[a,l]P is an environmental PAH derived from combustion sources that has been detected in particulate matter (PM) and tar samples (Schubert et al., 2003; Bergvall and Westerholm, 2007). DMBA, DMBA-diol, and DB[a,l]P all produced concentration-dependent suppression in the TDAR response in C57Bl/6J mice spleen cells. At the lowest concentration of DMBA tested (0.1 µM) there was a 30% suppression in TDAR compared to DMSO vehicle control, whereas DMBA-diol and DB[a,l]P produced nearly the same level of suppression at a concentration of 0.001 µM. These results indicate that DMBA-diol is 30–100 times more potent than the parent compound in producing immunosuppression in our system. These studies also demonstrate for the first time that DB[a,l]P, an environmentally relevant PAH, is extremely potent in inducing immunosuppression of the TDAR response.
Fig. 2.
Suppression of the SRBC TDAR by DMBA, DMBA-diol, or DB[a,l]P. No cytotoxicity was observed in these cultures. Data from a pool of three mouse spleens that were assayed in quadruplicate are shown as mean ± S.E.M. Statistically significant differences compared with 0.1% DMSO are indicated (*, p< 0.05).
Immunosuppressive Effects of NaAsO2 and Na2HAsO4 on the Spleen Cell TDAR Response in C57BL/6 mice
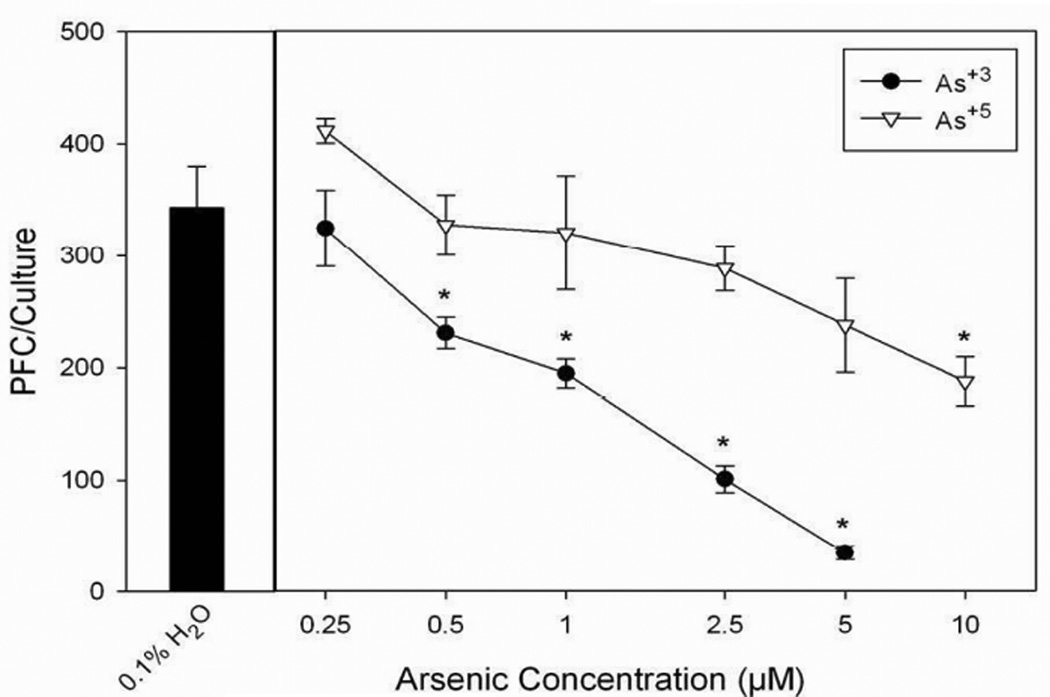
Sodium arsenite (As+3) and sodium arsenate (As+5) are important environmental forms of arsenic that have been shown to exert toxicity in mammals (Carter et al., 2003). The concentration-dependent immunotoxicity of As+3 and As+5 in C57BL/6J mice spleen cells was assessed using the TDAR assay (Figure 3). NaAsO2 (As+3) was found to suppress the TDAR at concentrations as low as 0.5 µM. Sodium arsenite was found to be at least 10-fold more suppressive to the TDAR than sodium arsenate (Na2HAsO4, As+5). As+3 is known to be more potent than As+5 in producing oxidative stress and reacting with protein sulfhydryls in many cell systems (Carter et al., 2003), which may provide an initial basis for understanding the differential toxicity of these agents.
Fig. 3.
Sodium arsenite (As+3) and sodium arsenate (As+5) treatment on the primary spleen cell humoral immune (IgM) response to SRBC TDAR as determined by PFC assay in male C57BL/6J mice examined in vitro. Spleen cells were immunized in vitro at the same time of treatment with H2O or As+3, As+5. No cytotoxicity was observed in these cultures. Data from a pool of three mouse spleens that were assayed in quadruplicate are shown as mean ± S.E.M. Statistically significant differences compared with 0.1% H2O are indicated (*, p< 0.05).
Synergistic Effect of Low Dose Arsenic and PAHs on the Spleen Cell TDAR Response in C57BL/6J mice
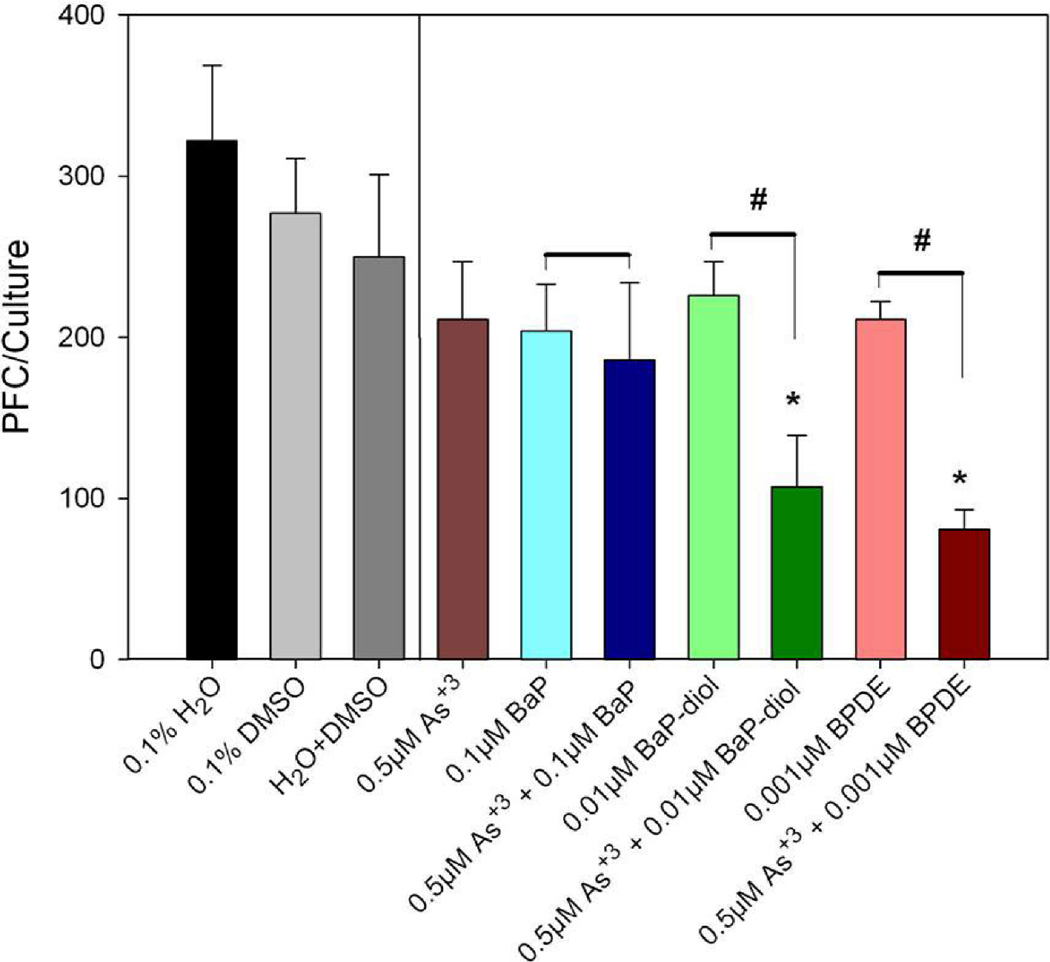
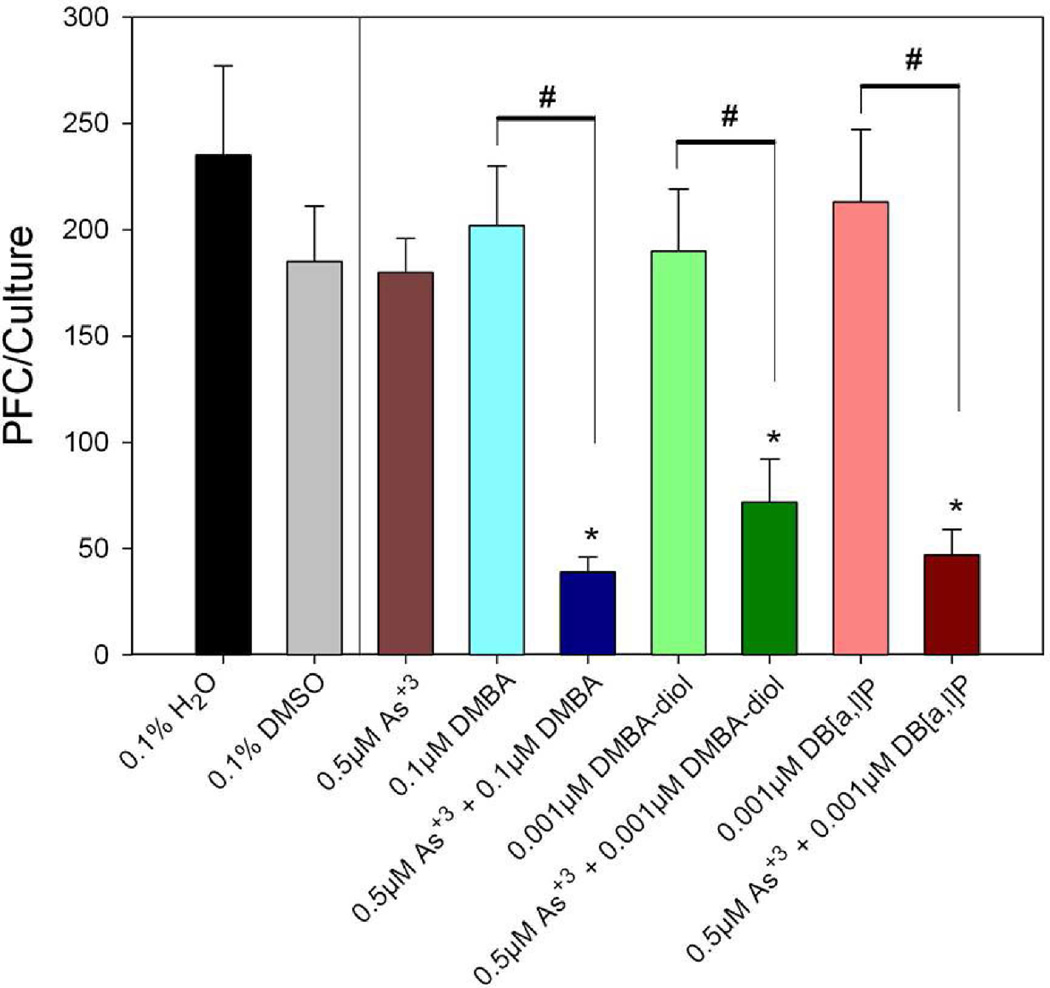
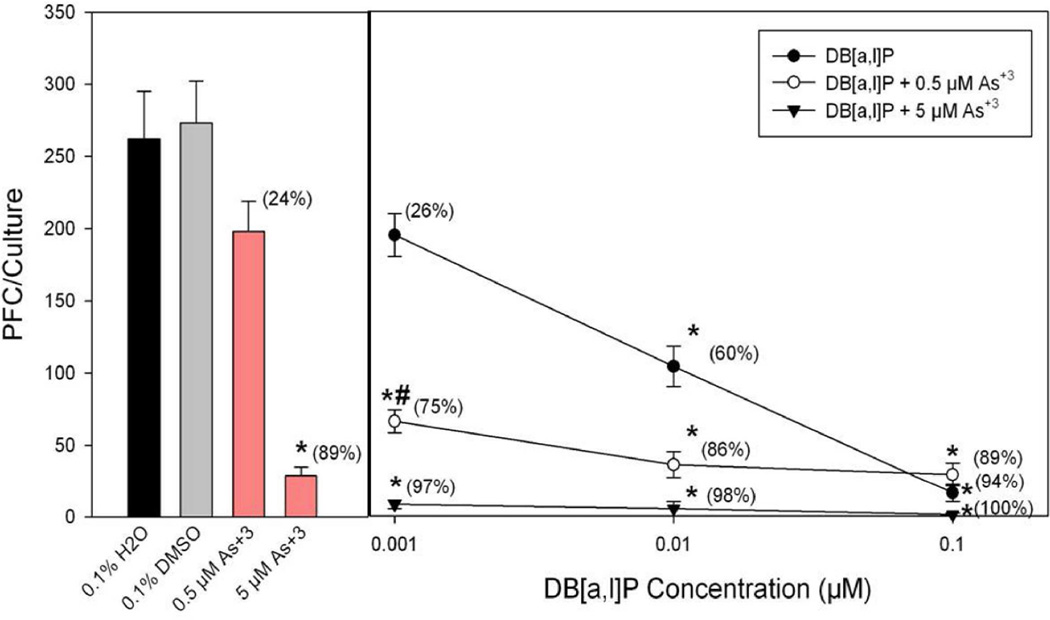
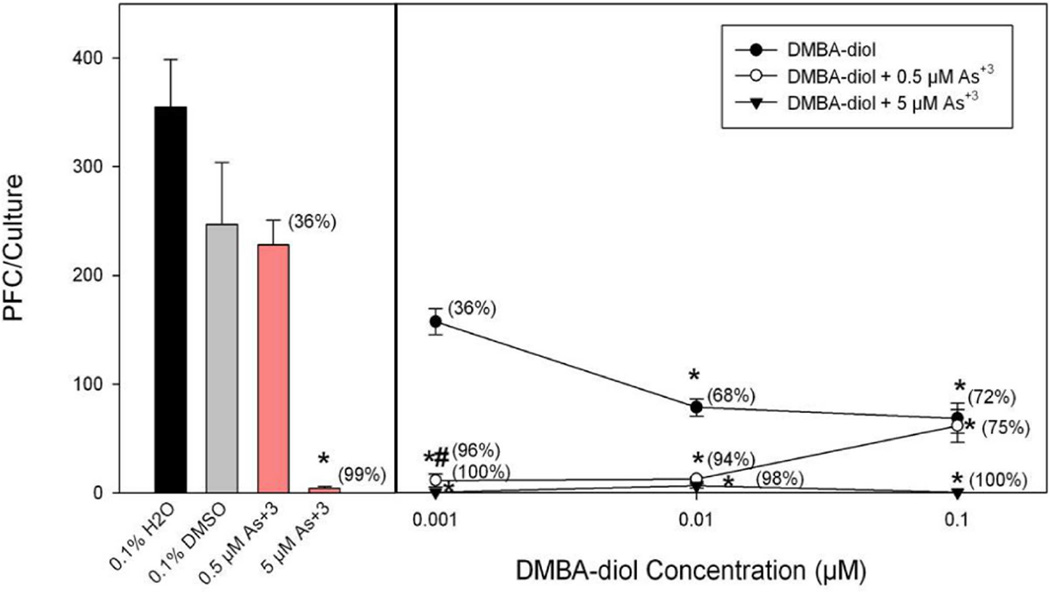
To evaluate effect of co-exposure of PAHs and arsenic, we chose in one experiment non-cytotoxic doses of BaP, BP-diol, and BPDE to combine with sodium arsenite to determine effects on the TDAR response. We found that spleen cell cultures co-treated with low doses of sodium arsenite and BaP-diol as well as sodium arsenite and BPDE demonstrated synergistic immunosuppressive effects (Figure 4). 0.01 µM BaP-diol and 0.001 µM BPDE did not produce significantly suppression by themselves. However, by co-treating with 0.5 µM of sodium arsenite, we found that significant immunosuppression was produced by BP-diol and BPDE. We performed a similar assessment of PAH and As+3 interaction in another experiment where we compared the effects of DMBA, DMBA-diol, and DB[a,l]P with or without 0.5 µM As+3 on the TDAR (Figure 5). The results shown in this figure demonstrated again that low dose PAH effects are greatly enhanced by a low dose of As+3. To further study the interactions between PAHs and As+3, we performed additional studies examining multiple doses of PAHs and As+3. In Figure 6, we found that 0.5 µM As+3 produced a greater than additive (synergistic) suppressive effect on the TDAR when combined with a low concentration (0.001 µM) of DB[a,l]P. A similar synergistic effects was seen between low concentrations of As+3 (0.5 µM) and DMBA-diol (0.001 µM) (Figure 7). The interactions between As+3 and PAHs at higher concentrations appear to be additive at PAH concentrations above 0.01 µM.
Fig. 4.
Effect of As+3 co-treatment with BaP, BaP-diol and BPDE on the in vitro primary spleen cell TDAR. Spleen cells were immunized in vitro at the same time of treatment with DMSO, As+3, and/or BaP, BaP-diol and BPDE. Data from a pool of three mouse spleens that were assayed in quadruplicate are shown as mean ± S.E.M. No cytotoxicity was observed in these cultures. *indicates a statistically significant difference compared with 0.1% H2O+ 0.1% DMSO (p< 0.05). #indicates a statistically significant effect of arsenite when added to PAHs compared to the PAH alone (p < 0.05). Data are representative of two experiments.
Fig. 5.
Effect of As+3 co-treatment with DMBA, DMBA-diol and DB[a,l]P on the primary spleen cell TDAR response to SRBC examined in vitro. Spleen cells were immunized in vitro at the same time of treatment with DMSO or As+3 and/or DMBA, DMBA-diol and DB[a,l]P. No cytotoxicity was observed in these cultures. Data from a pool of three mouse spleens that were assayed in quadruplicate are shown as mean ± S.E.M. *indicates a statistically significant difference compared with 0.1% H2O or 0.1% DMSO (p< 0.05). #indicates a statistically significant effect of arsenite when added to PAHs compared to the PAH alone (p < 0.05). Data are representative of two experiments.
Fig. 6.
Effect of As+3 co-treatment with DB[a,l]P on the primary spleen cell humoral immune (IgM) response to TDAR SRBC examined in vitro. Spleen cells were immunized in vitro at the same time of treatment with As+3 (0.5 or 5 µM in H2O) and/or DB[a,l]P (0.001 – 1 µM in DMSO). No cytotoxicity was observed in these cultures. Data from a pool of three mouse spleens that were assayed in quadruplicate are shown as mean ± S.E.M. *indicates a statistically significant effect of As+3 or PAHs compared to water control for As+3 or DMSO control for PAHs (p < 0.05). #indicates the synergistic interaction between As+3 and PAH as the suppression is greater than an additive effect.
Fig. 7.
Effect of As+3 co-treatment with DMBA-diol on the primary spleen cell humoral immune (IgM) response to SRBC examined in vitro. Spleen cells were immunized in vitro at the same time of treatment with As+3 (0.5 or 5 µM in H2O) and/or DMBA-diol (0.001 – 1 µM in DMSO). No cytotoxicity was observed in these cultures. Data from a pool of three mouse spleens that were assayed in quadruplicate are shown as mean ± S.E.M. *indicates a statistically significant effect of As+3 or PAHs compared to water control for As+3 or DMSO control for PAHs (p < 0.05). #indicates the synergistic interaction between As+3 and PAH as the suppression is greater than an additive effect.
Analysis of As+3 and PAH Interactions Using Western Blot Analysis of p53 Expression
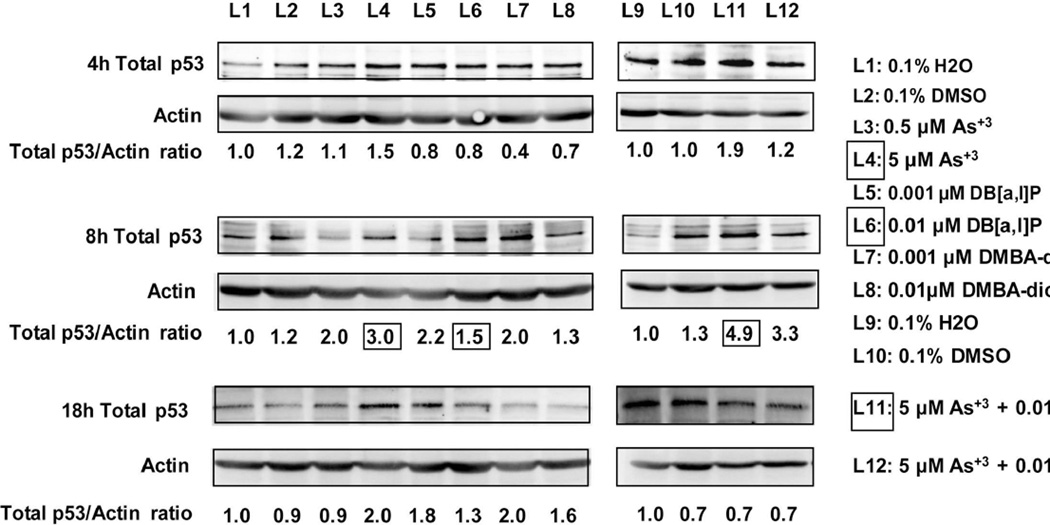
Previous studies by our lab have shown that p53 is required for the in vivo immunosuppression of the TDAR response (Gao et al., 2007b). Therefore, we were curious as to whether we could demonstrate an effect of As+3 and PAHs on the levels of p53 in cultured spleen cells in vitro. As shown in Figure 8, we found that 5 µM As+3 produced a dose- and time-dependent increase in p53 protein levels in murine spleen cells, with maximal effects observed 8 hrs after exposure. DB[a,l]P and DMBA-diol also produced a dose-and time-dependent increase in p53 protein, with maximal effects seen at 8 hrs. Co-exposure of murine spleen cells to 5 µM As+3 and 0.01 µM DB[a,l]P produced an increase in p53 proteins levels that appeared to be an additive effect based on the fold-inductions.
Fig. 8.
Western Blot analysis of As+3 and PAHs effects on whole cell lysate p53 protein levels in murine spleen cells treated in vitro at the indicated concentrations for 4, 8, or 18 hrs. Numbers shown at the bottom of each gel lane are the fold inductions for p53 protein normalized to actin and compared to controls (H2O for As+3 and DMSO for PAHs) and treated cells. The boxed numbers show the comparison for 5 µM As+3 induction (L4) of p53 at 8 hrs, as compared to 0.01 µM DB[a,l]P (L6) and the combination of both agents (L11).
Discussion
PAHs and arsenic coexist in many environments and it is likely that co-exposures and combined exposures occur in many humans throughout the world. As EPA classified human carcinogens, PAHs and arsenic have been intensively studied individually as chemical classes, but not in combination. We are not aware of any previous studies that have examined the immunosuppressive properties of these classes of chemical agents based on co-exposures. Our studies and others have shown that the most sensitive immune assay for assessment of PAH immunotoxicity is the TDAR. We have also found a strong agreement between the in vivo effects of PAHs and their effects in vitro. In vitro studies were employed in the present studies in order to examine the direct effects of PAHs and arsenic on murine spleen cells and to eliminate pharmacokinetic and other effects that may play a role in vivo immunotoxicity.
The results from these studies demonstrate concentration-dependent suppression of the TDAR response by BaP, DMBA, and DB[a,l]P. CYP-derived metabolites of BaP and DMBA were much more potent in producing immunosuppression than parent compounds. These results are consistent with our previous in vivo studies with DMBA demonstrating the requirement for CYP1B1 and mEH-dependent metabolism for immunosuppression of the TDAR (Gao et al., 2005, 2007a). It is well known that DMBA is more immunosuppressive than BaP in mice in vivo (White et al., 1985), which is likely explained by the fact that BaP induces significant CYP1A1 and 1A2 expression in the liver leading to mostly systemic elimination of tetrols (Uno et al., 2004, 2006)). The fact that BaP added directly to cultures is very immunosuppressive to spleen cells is in agreement with previous studies (White and Holsapple, 1984), and demonstrates that these cells have the ability to bioactivate the compound. Our studies suggest that the ultimate immunotoxic metabolites are likely BP-diol and BPDE, as they were found to have progressively increased immunotoxicity compared to the parent BaP.
DB[a,l]P is an interesting environmental PAH because it is found in PM air samples, in tar, and other environmental sources of potential exposure (Schubert et al., 2003; Bergvall and Westerholm, 2007). DB[a,l]P is one of the most potent carcinogenic PAHs that has been evaluated to date (Melendez-Colon et al., 1999; Castro et al., 2008). DMBA and DB[a,l]P are both activated by CYP1B1 (Buters et al., 2002) as well as by a radical cation pathway (Cavalieri and Rogan, 1995). Based on the known genotoxicity of DB[a,l]P, we predicted that it would be a potent immunotoxicant. Results from the present studies show that DB[a,l]P did indeed produce potent immunosuppression of the TDAR with a no-effect level below 0.001 µM. These findings warrant further investigation in vivo as well as a more complete characterization of the types of immunotoxicities and potential health effects of environmental exposure.
In the present studies, we established the concentration-response relationships for various PAHs in the in vitro TDAR assay and evaluated the potential interactions between PAHs and inorganic arsenic. Arsenic is a widely distributed contaminant in the environment. Immune suppression by arsenic has been observed in previous studies in both animal models and in humans living in arsenic contaminated areas. 50 mg/m3 and 1 mg/m3 inhalation exposures produced greater than 70% suppression of the TDAR (Burchiel et al., 2009). In previous studies, a significant decrease of T cell secreted cytokines and a marked dose-dependent suppression of Concanavalin A (Con A) induced T-cell proliferation was observed in arsenic-exposed individuals compared with the unexposed individuals (Soto-Peña et al., 2006). Chronic low-dose As exposure at the current U.S. drinking-water (10 ppb) standard has been reported to elicit effects on innate immune responses (Kozul et al., 2009). The mechanisms whereby arsenic alters adaptive and innate immunity have not been established.
Previous studies have shown that arsenic greatly potentiates the genotoxicity of BaP (Maier et al., 2002). DNA adduct levels in mouse skin and lung produced by BaP were found to be increased 8-fold by arsenic (Evans et al., 2004). These studies suggested that arsenic and BaP interact actively in vivo and in vitro. While the mechanism for arsenic and PAH interaction remains unexplained, these findings provide important rationale for the present study. A further justification for evaluating PAH-arsenic co-exposures is that recent studies have demonstrated that PAHs exert their immunotoxicity largely through the induction of genotoxic pathways (Gao et al., 2005, 2007a,b). PAHs form bulky adducts and DNA strand breaks that are sensed by ATM and ATR leading to the induction of p53 and subsequent cell cycle blockade and apoptosis which leads to immune suppression (Gao et al., 2007b). Many cells have the ability to repair DNA adducts and oxidative damage through enzyme pathways that include poly(ADP-ribose) polymerase-1 (PARP). Recent studies by one of our laboratories have shown that arsenite inhibits the activity of PARP by displacing zinc from its zinc-finger domain (Ding et al., 2009). Thus, we hypothesize that arsenite may potentiate the immunotoxicity of PAHs, perhaps by modulating DNA repair processes. This hypothesis will be tested in future studies.
The results of the present studies clearly demonstrate low dose synergistic interactions between PAHs and arsenite in producing immunosuppression as assessed using the TDAR. We also found that both As+3 and PAHs increase p53 expression in cultured murine spleen cells. The effects of co-exposure to As+3 and PAHs did not appear to be synergistic at the concentrations that we studied. However, p53 does appear to be involved in immunotoxicity produced by both classes of chemicals. Therefore, future studies are necessary to resolve the nature of the low dose synergy between As+3 and PAHs as well as to determine the target cells and mechanism(s) of action of these agents. In summary, the present studies are noteworthy because they suggest that extremely low and environmentally relevant co-exposures to arsenic and PAHs may impair host immunity.
Acknowledgments
This work was supported in part by a pilot grant from the UNM HSC Environmental Health Signature Program. Q.L. was supported by a graduate fellowship from the UNM College of Pharmacy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bergvall C, Westerholm R. Identification and determination of highly carcinogenic dibenzopyrene isomers in air particulate samples from a street canyon, a rooftop, and a subway station in Stockholm. Environ SciTech. 2007;41:731–737. doi: 10.1021/es062232p. [DOI] [PubMed] [Google Scholar]
- Burchiel SW, Davis DA, Gomez MP, Montano RM, Barton SL, Seamer LC. Inhibition of lymphocyte activation in splenic and gut-associated lymphoid tissues following oral exposure of mice to 7,12-dimethylbenz[a]anthracene. Toxicol Appl Pharmacol. 1990;105:434–442. doi: 10.1016/0041-008x(90)90147-m. [DOI] [PubMed] [Google Scholar]
- Burchiel SW, Davis DA, Ray SD, Archuleta MM, Thilsted JP, Corcoran GB. DMBA-induced cytotoxicity in lymphoid and nonlymphoid organs of B6C3F1 mice: relation of cell death to target cell intracellular calcium and DNA damage. Toxicol Appl Pharmacol. 1992;113:126–132. doi: 10.1016/0041-008x(92)90016-l. [DOI] [PubMed] [Google Scholar]
- Burchiel SW, Mitchell LA, Lauer FT, Sun X, McDonald JD, Hudson LG, Liu KJ. Immunotoxicity and Bisodistribution Analysis of Arsenic Trioxide in C57Bl/6 Mice Following a Two-Week Inhalation Exposure. Toxicol Appl Pharmacol. 2009;241:253–259. doi: 10.1016/j.taap.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns LA, Sikorski EE, Saady JJ, Munson AE. Evidence for arsenic as the immunosuppressive component of gallium arsenide. Toxicol Appl Pharmacol. 1991;110:157–169. doi: 10.1016/0041-008x(91)90298-s. [DOI] [PubMed] [Google Scholar]
- Buters JT, Mahadevan B, Quintanilla-Martinez L, Gonzalez FJ, Greim H, Baird WM, Luch A. Cytochrome P450 1B1 determines susceptibility to dibenzo[a,l]pyrene-induced tumor formation. Chem Res Toxicol. 2002;15:1127–1135. doi: 10.1021/tx020017q. [DOI] [PubMed] [Google Scholar]
- Castro DJ, Löhr CV, Fischer KA, Pereira CB, Williams DE. Lymphoma and lung cancer in offspring born to pregnant mice dosed with dibenzo[a,l]pyrene: the importance of in utero vs. lactational exposure. Toxicol Appl Pharmacol. 2008;233:454–458. doi: 10.1016/j.taap.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter DE, Aposhian HV, Gandolfi AJ. The metabolism of inorganic arsenic oxides, gallium arsenide, and arsine: a toxicochemical review. Toxicol Appl Pharmacol. 2003;193:309–334. doi: 10.1016/j.taap.2003.07.009. [DOI] [PubMed] [Google Scholar]
- Cavalieri EL, Rogan EG. Central role of radical cations in metabolic activation of polycyclic aromatic hydrocarbons. Xenobiotica. 1995;25:677–688. doi: 10.3109/00498259509061885. [DOI] [PubMed] [Google Scholar]
- Cohen SM, Arnold LL, Eldan M, Lewis AS, Beck BD. Methylated arsenicals: the implications of metabolism and carcinogenicity studies in rodents to human risk assessment. Crit Rev Toxicol. 2006;36:99–133. doi: 10.1080/10408440500534230. [DOI] [PubMed] [Google Scholar]
- Davila DR, Romero DL, Burchiel SW. Human T cells are highly sensitive to suppression of mitogenesis by polycyclic aromatic hydrocarbons and this effect is differentially reversed by alpha-naphthoflavone. Toxicol Appl Pharmacol. 1996;139:333–341. doi: 10.1006/taap.1996.0173. [DOI] [PubMed] [Google Scholar]
- Ding W, Liu W, Cooper KL, Qin XJ, de Souza Bergo PL, Hudson LG, Liu KJ. Inhibition of poly(ADP-ribose) polymerase-1 by arsenite interferes with repair of oxidative DNA damage. J Biol Chem. 2009;284:6809–6817. doi: 10.1074/jbc.M805566200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H, Dalton TP, Miller ML, Chen Y, Uno S, Shi Z, Shertzer HG, Bansal S, Avadhani NG, Nebert DW. Knock-in mouse lines expressing either mitochondrial or microsomal CYP1A1: differing responses to dietary benzo[a]pyrene as proof of principle. Mol Pharmacol. 2009;75:555–567. doi: 10.1124/mol.108.051888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans CD, LaDow K, Schumann BL, Savage RE, Caruso J, Vonderheide A, Succop P, Talaska G. Effect of arsenic on benzo[a]pyrene DNA adduct levels in mouse skin and lung. Carcinogenesis. 2004;25:493–497. doi: 10.1093/carcin/bgg199. [DOI] [PubMed] [Google Scholar]
- Gao J, Lauer FT, Dunaway S, Burchiel SW. Cytochrome P450 1B1 Is Required for 7,12-Dimethylbenz(a)-anthracene (DMBA) Induced Spleen Cell Immunotoxicity. Toxicol Sci. 2005;86:68–74. doi: 10.1093/toxsci/kfi176. [DOI] [PubMed] [Google Scholar]
- Gao J, Lauer FT, Mitchell LA, Burchiel SW. Microsomal expoxide hydrolase Is required for 7,12-dimethylbenz[a]-anthracene (DMBA)–induced immunotoxicity in mice. Toxicol Sci. 2007a;98:137–144. doi: 10.1093/toxsci/kfm089. [DOI] [PubMed] [Google Scholar]
- Gao J, Mitchell LA, Lauer FT, Burchiel SW. p53 and ATM/ATR regulate 7,12-dimethylbenz[a]anthracene-induced immunosuppression. Mol Pharmacol. 2007b;73:137–146. doi: 10.1124/mol.107.039230. [DOI] [PubMed] [Google Scholar]
- Kozul CD, Ely KH, Enelow RI, Hamilton JW. Low-dose arsenic compromises the immune response to influenza A infection in vivo. Environ Health Perspect. 2009;117:1441–1447. doi: 10.1289/ehp.0900911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau HH, Coffing SL, Lee H, Harvey RG, Baird WM. Stereoselectivity of activation of 7,12-dimethylbenz[a]anthracene-3,4-dihydrodiol to the anti-diol epoxide metabolite in a human mammary carcinoma MCF-7 cell-mediated V79 cell mutation assay. Chem Res Toxicol. 1995;8:970–978. doi: 10.1021/tx00049a011. [DOI] [PubMed] [Google Scholar]
- Li Y, Liu J, Cao Z, Lin C, Yang Z. Spatial distribution and health risk of heavy metals and polycyclic aromatic hydrocarbons (PAHs) in the water of the Luanhe River Basin, China. Environ Monit Assess. 2009 Mar 3; doi: 10.1007/s10661-009-0811-2. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Maier A, Schumann BL, Chang X, Talaska G, Puga A. Arsenic co-exposure potentiates benzo[a]pyrene genotoxicity. Mutation Res. 2002;517:101–111. doi: 10.1016/s1383-5718(02)00057-8. [DOI] [PubMed] [Google Scholar]
- Melendez-Colon VJ, Luch A, Seidel A, Baird WM. Cancer initiation by polycyclic aromatic hydrocarbons results from formation of stable DNA adducts rather than apurinic sites. Carcinogenesis. 1999;20:1885–1891. doi: 10.1093/carcin/20.10.1885. [DOI] [PubMed] [Google Scholar]
- Roberts JW, Wallace LA, Camann DE, Dickey P, Gilbert SG, Lewis RG, Takaro TK. Monitoring and reducing exposure of infants to pollutants in house dust. Rev Environ Contam Toxicol. 2009;201:1–39. doi: 10.1007/978-1-4419-0032-6_1. Review. [DOI] [PubMed] [Google Scholar]
- Rossman TG. Mechanism of arsenic carcinogenesis: an integrated approach. Mutation Res. 2003;533:37–65. doi: 10.1016/j.mrfmmm.2003.07.009. [DOI] [PubMed] [Google Scholar]
- Schubert, P Schantz MM, Sander LC, Wise SA. Determination of polycyclic aromatic hydrocarbons with molecular weight 300 and 302 in environmental-matrix standard reference materials by gas chromatography/mass spectrometry. Anal Chem. 2003;75:234–246. doi: 10.1021/ac0259111. [DOI] [PubMed] [Google Scholar]
- Sikorski EE, McCay JA, White KI, Bradley SG, Munson AE. Immunotoxicity of the semiconductor gallium arsenide in female B6C3F1 mice. Fundam Appl Toxicol. 1989;13:843–858. doi: 10.1016/0272-0590(89)90338-2. [DOI] [PubMed] [Google Scholar]
- Snow ET, Sykora P, Durham TR, Klein CB. Arsenic, mode of action at biologically plausible low doses: What are the implications for low dose cancer risk? Toxicol Appl Pharmacol. 2005;207:557–564. doi: 10.1016/j.taap.2005.01.048. [DOI] [PubMed] [Google Scholar]
- Soto-Peña GA, Luna AL, Acosta-Saavedra L, Conde-Moo P, López-Carrillo L, Cebrián ME, Bastida M, Calderón-Aranda ES, Vega L. Assessment of lymphocyte subpopulations and cytokine secretion in children exposed to arsenic. FASEB J. 2006;20:779–781. doi: 10.1096/fj.05-4860fje. [DOI] [PubMed] [Google Scholar]
- Uno S, Dalton TP, Derkenne S, Curran CP, Miller ML, Shertzer HG, Nebert DW. Oral exposure to benzo[a]pyrene in the mouse: detoxication by inducible cytochrome P450 is more important than metabolic activation. Mol Pharmacol. 2004;65:1225–1237. doi: 10.1124/mol.65.5.1225. [DOI] [PubMed] [Google Scholar]
- Uno S, Dalton TP, Dragin N, Curran CP, Derkenne S, Miller ML, Shertzer HG, Gonzalez FJ, Nebert DW. Oral benzo[a]pyrene in Cyp1 knockout mouse lines: CYP1A1 important in detoxication, CYP1B1 metabolism required for immune damage independent of total-body burden and clearance rate. Mol Pharmacol. 2006;69:1103–1114. doi: 10.1124/mol.105.021501. [DOI] [PubMed] [Google Scholar]
- White KL, Jr, Holsapple MP. Direct suppression of in vitro antibody production by mouse spleen cells by the carcinogen benzo(a)pyrene but not by the noncarcinogenic congener benzo(e)pyrene. Cancer Res. 1984;44:3388–3393. [PubMed] [Google Scholar]
- White KL, Jr, Lysy HH, Holsapple MP. Immunosuppression by polycyclic aromatic hydrocarbons: a structure-activity relationship in B6C3F1 and DBA/2 mice. Immunopharmacology. 1985;9:155–164. doi: 10.1016/0162-3109(85)90011-6. [DOI] [PubMed] [Google Scholar]
- White KL, Musgrove DL, Brown RD. The sheep erythrocyte T-dependent antibody Response (TDAR) Methods Mol Biol. 2010;598:173–184. doi: 10.1007/978-1-60761-401-2_12. [DOI] [PubMed] [Google Scholar]