Abstract
Objective
Osteoarthritis (OA) is a common chronic disease for which disease-modifying therapies are not currently available. Studies to seek new targets for slowing the progress of OA rely on mouse models, but these do not allow for longitudinal monitoring of disease development. This study was undertaken to determine whether gait can be used to measure disease severity in the STR/Ort mouse model of spontaneous OA and whether gait changes are related to OA joint pain.
Methods
Gait was monitored using a treadmill-based video system. Correlations between OA severity and gait at 3 treadmill speeds were assessed in STR/Ort mice. Gait and pain behaviors of STR/Ort mice and control CBA mice were analyzed longitudinally, with monthly assessments.
Results
The best speed to identify paw area changes associated with OA severity in STR/Ort mice was found to be 17 cm · seconds−1. Paw area was modified with age in CBA and STR/Ort mice, but this began earlier in STR/Ort mice and correlated with the onset of OA at 20 weeks of age. In addition, task noncompliance appeared at 20 weeks. Surprisingly, STR/Ort mice did not show any signs of pain with OA development, even when treated with the opioid antagonist naloxone, but did exhibit normal pain behaviors in response to complete Freund's adjuvant–induced arthritis.
Conclusion
The present results identify an animal model in which OA severity and OA pain can be studied in isolation from one another. The findings suggest that paw area and treadmill noncompliance may be useful tools to longitudinally monitor nonpainful OA development in STR/Ort mice. This will help in providing a noninvasive means of assessing new therapies to slow the progression of OA.
Osteoarthritis (OA), the most common chronic joint disease (affecting >25% of persons age ≥60 years), remains without effective disease-modifying therapy. Animal models have been used to study OA, with mouse studies currently central in providing the paradigms aimed at identifying new treatment targets. Mouse models of spontaneous and mechanically induced OA each have similarities to human disease (1–4). Beginning at ∼20 weeks of age, male STR/Ort mice develop a natural OA (5,6) that engages mechanisms resembling those of human OA, with loss of articular cartilage proteoglycan, progressive articular cartilage degeneration (6), osteophytogenesis, and subchondral bone thickening (1,7–9). The usefulness of these mice for studying OA is, however, limited by their small joint size and by the paucity of noninvasive tools for longitudinal monitoring of disease.
Methods for noninvasive monitoring of OA severity would enhance the utility of STR/Ort mice for the study of OA and enhance efforts to develop new therapies. Specific gait modifications have been linked with OA severity and knee pain in humans (10–13), and advances in monitoring of pain-associated changes in murine OA would be highly advantageous. Indeed, recent studies have demonstrated that measures of gait represent a simple and objective method for monitoring both arthritis development and treatment response (14,15).
With a view toward extending these analyses to mice with spontaneous OA, we have addressed whether gait changes, which may reflect pain, can be used to monitor OA progression in STR/Ort mice. To our knowledge, few such in-depth studies focusing on gait measurement in mice have been performed previously (16). We used treadmill-based analyses to determine whether biomechanical characteristics of gait in STR/Ort mice are predictive of OA onset or progression and whether these changes are related to OA joint pain. We found that paw area was the best predictor of the severity of spontaneous OA in STR/Ort mice. Surprisingly, we also observed that STR/Ort mice with OA did not exhibit significant pain behaviors and that treadmill task noncompliance (refusal to run) was another potential noninvasive measure of advanced OA in this naturally OA-prone strain. Our findings suggest that disabling, likely structural, joint changes, as opposed to pain, are responsible for modifications in gait seen during OA development in these mice and that reduced paw area and treadmill task noncompliance are the most effective measures of OA onset and progression. They also highlight the possible identification of the STR/Ort mouse as a new animal model to enable study of mechanisms involved in the development of severe OA without the complicating factor of associated pain.
MATERIALS AND METHODS
Animals
Male CBA mice (Charles River) and STR/Ort mice (bred at our institution) were kept in polypropylene cages, subjected to 12-hour light/dark cycles at 21°C (±2°C), and fed standard RM1 maintenance diet (Special Diet Services) ad libitum. All procedures were in compliance with the Animals (Scientific Procedures) Act (1986) and were approved by the local ethics committee.
CBA is a member of the parental stock from which STR/Ort mice were originally derived (17,18), and age-matched CBA mice were included as a non–OA-prone, healthy joint aging control group. In study 1, 33 STR/Ort mice ages 18–56 weeks (encompassing all grades of OA) were used to determine the optimum treadmill speed (13, 17, or 24 cm · seconds−1) to maximize gait differences at distinct degrees of OA severity and for preliminary assessments prior to longitudinal analyses; data on all animals were included regardless of possible noncompliance with running at some speeds. Mice were killed by cervical dislocation, and knees were fixed, decalcified (Immunocal), and 6-μm coronal wax sections cut. Sections (120-μm intervals) across each entire joint were stained with toluidine blue, and OA severity was graded using the Osteoarthritis Research Society International scoring system (19). In study 2, 13 STR/Ort mice ages 20–25 weeks were used to determine the frequency of task noncompliance at treadmill speeds of 17 and 24 cm · seconds−1. In study 3, 7 STR/Ort mice age 16 weeks were used to test the reproducibility of gait recordings on 10 separate occasions over 2 weeks. In study 4, 22 STR/Ort mice and 20 CBA mice were used to longitudinally analyze gait (assessed monthly) between the ages of 8 weeks and 36 weeks. In study 5, 13 STR/Ort mice and 10 CBA mice were used to assess pain behaviors between the ages of 16 weeks and 36 weeks.
Gait analysis
Gait was recorded using a DigiGait imaging system (Mouse Specifics) (14). Briefly, mice ran on a transparent flat treadmill at a specific speed, while a video camera captured ventral images. Animals ran for a maximum of 30 seconds for each measurement, with segments of 5 seconds (which corresponded to >10 consecutive strides) used for analysis. No habituation procedures were used for these studies at any time. DigiGait image analysis software automatically defines each paw area, generates waveforms to describe advance/retreat of each limb in consecutive strides, and identifies periods of time when each paw is in treadmill contact as stance phase, and intervening periods as swing phase. Postural and kinematic gait measurements are also calculated, including stride time, stride length, and paw area. Brake and propel times were defined as the times before and after maximal paw area during the stance phase, and paw angle was represented as the angle of the paw relative to the long axis (14). The symmetry index is defined as the absolute value of the difference between contralateral hind limbs divided by their average. After investigation of a range of speeds (study 1) and reproducibility (study 2), treadmill speed for subsequent studies (study 3) was set at 17 cm · seconds−1.
Mouse treadmill task noncompliance (i.e., refusal to undertake or complete the treadmill running task, which would be observed as inability or unwillingness of a mouse to take more than 2 consecutive strides) was also recorded. Noncompliance, or “dropout,” was irreversible; reluctance of any STR/Ort mouse to complete the task meant that this particular mouse would not comply on any further occasions upon which opportunity was afforded (i.e., a trial with noncompliance would not be followed at any time in the future by a trial in which compliance was achieved).
Measurement of pain-associated behavior
Pain behaviors (20) in the hind limbs of 16–36-week-old STR/Ort mice (n = 13) and CBA mice (n = 10) (study 5) were assessed. Briefly, mechanical allodynia was assessed by placing mice in a Perspex chamber with a metal grid floor and the paw's plantar surface (left and right hind paws) touched for <6 seconds with von Frey filaments in ascending order of force (0.04–1.4g). A positive response was recorded if the paw was sharply withdrawn or if flinching was observed upon filament removal. Once a positive withdrawal response was established, the paw was retested starting with the next descending von Frey filament until no response occurred; the testing of responses to von Frey filaments of ascending and descending force was then repeated until a reproducible threshold force was determined. The results were therefore based on at least 2 rounds of measurements. The smallest force eliciting a response was recorded as the paw withdrawal threshold (20,21). Mechanical hyperalgesia (paw pressure) was assessed by applying increasing pressure stimulus on the hind paw's dorsal surface using an analgesymeter (no. 7200; Ugo Basile) and recording the degree of pressure at which paw withdrawal was induced (22). Cold sensitivity was assessed using a 10°C cold-plate (Ugo Basile), with time until paw withdrawal recorded (23).
The assessment of audible vocalizations has been found to be a valid method for determining knee hypersensitivity. For example, in a study of a surgical model of OA in C57BL/6 mice with the operator being blinded with regard to the clinical status of the groups under study, the number of audible vocalizations was found to be consistent with OA pain behaviors such as mechanical hypersensitivity (paw pressure) and cold sensitivity (20). In the present study the number of audible vocalizations was recorded during 10 repeated compressions of the knee between the operator's thumb and forefinger with moderate force. Tests on all animals was performed by the same operator. As the strains in the current study are readily identifiable by their skin coloration, blinding of the operator was not possible.
To define relationships between pain and gait changes, carprofen (5 mg/kg) was administered intraperitoneally for 3 consecutive days (24) and gait analyzed on the third day. To define whether absence of expected pain behaviors was due to masking effects of endogenous opioids and to reveal likely maxima in pain-related behavior, naloxone hydrochloride (2.5 mg/kg; Sigma-Aldrich) or vehicle was administered intraperitoneally to randomly allocated mice. Behavioral readings were recorded before and 60 minutes after this treatment. To test whether STR/Ort mice developed abnormal sensitivities in a known joint pain model, inflammatory knee arthritis was induced in 40–43-week-old STR/Ort mice (n = 3) by intraarticular injection of Freund's complete adjuvant (CFA; Fisher Scientific) (10 μl; 0.1% [weight/volume] in mineral oil) and pain measures assessed 72 hours later.
Statistical analysis
Data are shown as the mean ± SEM. Groups of STR/Ort mice in study 1 were compared by one-way analysis (ANOVA) followed by Fisher's post hoc analysis of least significant difference and Holm's adjustment for multiple testing. Results obtained in studies 3 and 5 were analyzed by repeated-measures ANOVA followed by Tukey's post hoc comparison. Effects of naloxone (study 5) were compared by unpaired t-test. For longitudinal studies of gait in STR/Ort and CBA mice (study 4), principal components analysis (PCA), a dimension reduction technique, was used to summarize 41 correlated measurements into a few uncorrelated principal components across both strains. Heatmaps were used to depict contributions (loading) of original gait measurements to principal components, and linear mixed-effects models were used to assess whether principal components differed between strains, by age, and strain–age interaction. First-degree autoregressive covariance structure was used to account for correlation in repeated measurements. Discriminant analysis, a data classification technique, is a procedure that uses information on several variables and predicts the group to which a subject belongs based on those variables. Discriminant analysis was used to determine the linear combinations of gait measurements that best discriminate age at “dropout” from the treadmill task for the STR/Ort mice (no dropout was evident in the non–OA-prone CBA mice). Unpaired t-tests and repeated-measures ANOVA were performed using GraphPad Prism version 6. PCA, discriminant analysis, and linear mixed-effects analysis were carried out using SPSS version 20. P values less than 0.05 were considered significant.
RESULTS
Association of modified paw area and paw angle with OA severity
Before longitudinal analyses were conducted, OA severity in the joints of 18–56-week-old STR/Ort mice (n = 33) was graded after analysis of gait at various treadmill speeds (13, 17, and 24 cm · seconds−1) (study 1). As expected, higher treadmill speeds led to decreased brake, propel, stance, and stride times and increased stride length. After allocation of mice to 1 of 3 groups based on OA severity (low/low [both left and right limbs with a grade of ≤4], high/high [both limbs with a grade of ≥5], or low/high [one limb with grade of ≥5 and the contralateral limb with a grade of ≤4]), experiments using treadmill speeds of 17 cm · seconds−1 revealed significant differences in paw area according to OA severity and speeds of 24 cm · seconds−1 revealed significant differences in swing time according to OA severity, whereas no significant difference in gait was observed at speeds of 13 cm · seconds−1 (Table1). Due to the high number of statistical tests performed on the data shown in Table1, Holm's adjustment for multiple testing was performed; after this adjustment, the only parameter that remained significantly different between the groups categorized by disease severity was paw area at 17 cm · seconds−1. The data, however, suggest that higher treadmill speeds will provide better detection of OA-related gait changes and that paw area is likely a particularly sensitive parameter. In addition, the grouping of mice and stringent statistical analysis revealed no significant asymmetries in gait at any of the speeds tested (Table1).
Table 1.
Parameters of hind limb gait and symmetry index according to OA severity, in STR/Ort mice performing treadmill tasks at 3 speeds*
| Hind limb gait measurement, average of both limbs |
Symmetry index† |
|||||
|---|---|---|---|---|---|---|
| Treadmill speed, parameter | Low/low severity | Low/high severity | High/high severity | Low/low severity | Low/high severity | High/high severity |
| 24 cm/second (n = 3, 6, 8)‡ | ||||||
| Swing, msec | 91 ± 4 | 72 ± 5§ | 87 ± 3 | 0.02 ± 0.01 | 0.09 ± 0.03 | 0.04 ± 0.01 |
| Brake, msec | 41 ± 8 | 32 ± 3 | 33 ± 2 | 0.08 ± 0.04 | 0.16 ± 0.05 | 0.19 ± 0.05 |
| Propel, msec | 130 ± 5 | 122 ± 10 | 125 ± 6 | 0.01 ± 0.01 | 0.06 ± 0.01 | 0.04 ± 0.02 |
| Stance, msec | 172 ± 3 | 155 ± 12 | 157 ± 6 | 0.02 ± 0.01 | 0.06 ± 0.02 | 0.07 ± 0.02 |
| Stride, msec | 263 ± 6 | 227 ± 17 | 245 ± 8 | 0.01 ± 0.00 | 0.07 ± 0.02 | 0.04 ± 0.01 |
| Paw area, cm2 | 0.99 ± 0.02 | 0.89 ± 0.05 | 0.76 ± 0.08 | 0.03 ± 0.01 | 0.09 ± 0.03 | 0.12 ± 0.02 |
| Paw angle, degrees | 13.8 ± 3.2 | 14.0 ± 1.2 | 12.5 ± 1.3 | 0.12 ± 0.03 | 0.31 ± 0.14 | 0.18 ± 0.06 |
| 17 cm/second (n = 4, 9, 9)‡ | ||||||
| Swing, msec | 95 ± 6 | 85 ± 4 | 86 ± 4 | 0.09 ± 0.03 | 0.05 ± 0.02 | 0.07 ± 0.03 |
| Brake, msec | 38 ± 7 | 41 ± 3 | 48 ± 3 | 0.13 ± 0.05 | 0.22 ± 0.04 | 0.22 ± 0.04 |
| Propel, msec | 126 ± 34 | 172 ± 13 | 161 ± 6 | 0.08 ± 0.04 | 0.09 ± 0.03 | 0.06 ± 0.02 |
| Stance, msec | 164 ± 40 | 212 ± 15 | 209 ± 6 | 0.08 ± 0.04 | 0.09 ± 0.03 | 0.06 ± 0.01 |
| Stride, msec | 258 ± 41 | 297 ± 17 | 295 ± 9 | 0.08 ± 0.03 | 0.07 ± 0.02 | 0.04 ± 0.01 |
| Paw area, cm2 | 0.50 ± 0.10¶ | 0.82 ± 0.05 | 0.71 ± 0.04 | 0.08 ± 0.04 | 0.13 ± 0.04 | 0.12 ± 0.04 |
| Paw angle, degrees | 10.7 ± 4.5 | 11.7 ± 1.6 | 12.9 ± 2.2 | 0.19 ± 0.07 | 0.25 ± 0.09 | 0.18 ± 0.05 |
| 13 cm/second (n = 5, 9, 9)‡ | ||||||
| Swing, msec | 89 ± 9 | 81 ± 7 | 94 ± 9 | 0.10 ± 0.04 | 0.07 ± 0.02 | 0.10 ± 0.02 |
| Brake, msec | 50 ± 9 | 47 ± 5 | 50 ± 5 | 0.19 ± 0.04 | 0.25 ± 0.03 | 0.19 ± 0.04 |
| Propel, msec | 201 ± 24 | 184 ± 20 | 197 ± 16 | 0.12 ± 0.02 | 0.14 ± 0.04 | 0.08 ± 0.03 |
| Stance, msec | 251 ± 30 | 231 ± 23 | 247 ± 16 | 0.07 ± 0.02 | 0.11 ± 0.04 | 0.08 ± 0.02 |
| Stride, msec | 341 ± 38 | 313 ± 29 | 341 ± 23 | 0.07 ± 0.03 | 0.09 ± 0.03 | 0.06 ± 0.02 |
| Paw area, cm2 | 0.84 ± 0.12 | 0.84 ± 0.07 | 0.93 ± 0.04 | 0.09 ± 0.03 | 0.11 ± 0.02 | 0.16 ± 0.03 |
| Paw angle, degrees | 12.8 ± 1.3 | 10.2 ± 2.0 | 13.0 ± 5.1 | 0.36 ± 0.16 | 0.39 ± 0.12 | 0.25 ± 0.06 |
Osteoarthritis (OA) severity in paired hind limbs was retrospectively graded as low/low (both left and right limbs with an OA grade of ≤4), high/high (both limbs with a grade of ≥5), or low/high (one limb with a grade of ≥5 and contralateral limb with a grade of ≤4). Values are the mean ± SEM.
Defined as the absolute value of the difference between contralateral hind limbs divided by their average.
Number of mice in the low/low, low/high, and high/high OA severity groups, respectively.
P = 0.02 versus the low/low and high/high severity groups by one-way analysis of variance with Fisher's least significant difference test for post hoc comparison.
P = 0.007 versus the low/high and high/high severity groups by one-way analysis of variance with Fisher's least significant difference analysis for post hoc comparison; significance remained after Holm's adjustment for multiple testing.
STR/Ort mice were more task-compliant at treadmill speeds of 17 cm · seconds−1 compared to higher speeds. The number of STR/Ort mice (20–25 weeks-old; n = 13) that dropped out increased from 2 at speeds of 17 cm · seconds−1 to 8 at 24 cm · seconds−1 (study 2). Given this relative noncompliance at faster speeds and the gait changes associated with OA severity at 17 cm · seconds−1, further studies were performed at 17 cm · seconds−1. The validity of longitudinal gait examinations depends both on reproducibility and on the possible impact of each treadmill task on subsequent gait. We therefore measured gait at treadmill speeds of 17 cm · seconds−1 on 10 separate occasions over 2 weeks in 16-week-old STR/Ort mice (n = 7) and found that none of the parameters showed any significant changes (study 3; data not shown).
Gait analysis reveals paw area as the main parameter associated with OA in aging STR/Ort mice
Monthly treadmill task analysis in CBA and STR/Ort mice from age 8 weeks to age 36 weeks (study 4) demonstrated that CBA mice showed full compliance, whereas only 3 of 22 STR/Ort mice (14%) completed the task throughout the study period. Timing of the first dropout by STR/Ort mice coincided with OA onset at 20 weeks (5), suggesting a possible link with OA development. Discriminant analysis revealed that the gait of STR/Ort mice during the 2 months immediately prior to dropout deviated significantly from gait at earlier time points (discriminant score 1; mainly included paw area) (P < 0.0001) (Figure 1). Comparison of OA severity in the STR/Ort mice exhibiting early dropout versus those exhibiting late dropout (20–24 weeks versus 32–36 weeks) showed that age at dropout did not correlate with the OA grade at the end of the study (36 weeks).
Figure 1.
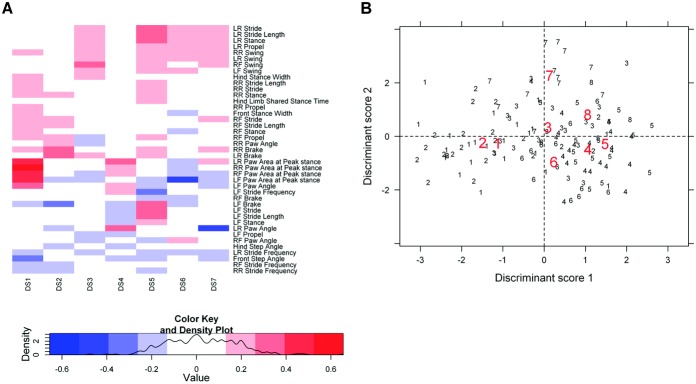
Link between gait and task noncompliance (treadmill task dropout) in osteoarthritis-prone STR/Ort mice, demonstrated by discriminant analysis. A, Heatmap representing the contribution (loading) of the 41 gait parameters to the first 7 discriminant scores (DS). This analysis demonstrates that paw area contributed the most to discriminant score 1. LR = left rear limb; RR = right rear limb; LF = left front limb; RF = right front limb. B, Scatterplot of the first 2 discriminant scores based on gait changes occurring in STR/Ort mice at each month immediately prior to the time of dropout. Modifications in gait (represented in discriminant score 1) occurred in the last 2 months preceding dropout from the treadmill task. Thus, group 1 (each animal marked with this number) represents the final gait measurements in the month before dropout, group 2 the second-to-last measurements before dropout (i.e., values from mice 2 months prior to dropout) and so on, up to group 8. Centroids for each of these groups are shown in red. The further apart the centroids, the more accurate the group classification. The last 2 records before dropout had lower discriminant score 1 values compared to the rest of the records.
PCA to assess whether gait changes in STR/Ort and CBA mice were associated with age showed that the first PC (PCA1) captured 39% of the variation in gait in both CBA and STR/Ort mice, but failed to reveal any age-related trend in gait (Figure 2B). PCA1 correlated most with stride, swing, stance, and propel times, stride length, and stride frequency (Figure 2A). These parameters were generally higher in STR/Ort mice compared to CBA mice (except for stride frequency, for which the opposite was the case), but did not change significantly with age. In addition, the symmetry index did not reveal any significant gait asymmetries with age in either strain of mice.
Figure 2.
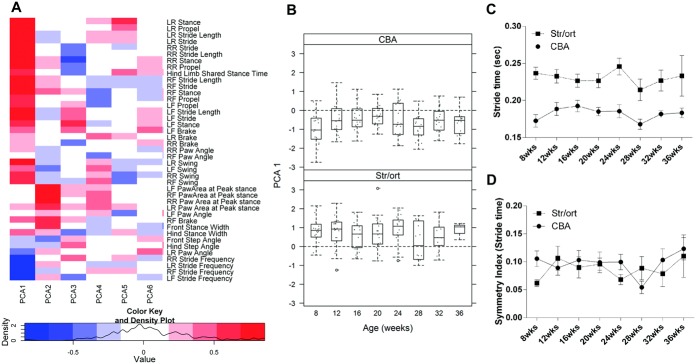
Longitudinal variation in the first principal component analysis (PCA1) and one of its individual components (stride time) is not associated with aging in CBA or STR/Ort mice. A, Heatmap representing the contribution (loading) of the 41 gait parameters to the first 6 principal components (PCA1–PCA6) across ages in CBA and STR/Ort mice. This analysis demonstrates that stride, swing, stance, and propel time and stride frequency contributed the most to PCA1 and that paw area contributed the most to PCA2. B, Changes in PCA1 in CBA mice and STR/Ort mice between 8 and 36 weeks of age. Significant differences were noted between strains, but differences between time points were not observed. Data are shown as box plots. Each box represents the 25th to 75th percentiles. Lines inside the boxes represent the median. Dashed lines outside the boxes represent the 10th and the 90th percentiles. Dots represent individual mice. Circles indicate outliers. C, Stride time (mean of the left limbs and right limbs of each animal) in CBA and STR/Ort mice. Stride times did not change significantly with aging, but were higher in STR/Ort mice than in CBA mice (P < 0.05) at all time points beginning at 8 weeks. D, Symmetry index for stride time in CBA and STR/Ort mice. Stride times did not change significantly with aging and did not differ between strains. Values in C and D are the mean ± SEM. For CBA mice, n = 20 at all time points; for STR/Ort mice, n = 22 at weeks 8, 12, and 16, n = 21 at week 20, n = 17 at week 24, n = 14 at week 28, n = 13 at week 32, and n = 3 at week 36. See Figure 1 for other definitions.
The second PC (PCA2) captured 11% of variation, and revealed significant age-related patterns in both strains (Figure 3A). In CBA mice, PCA2 remained stable until age 28 weeks and decreased significantly at 32–36 weeks. In contrast, in STR/Ort mice, PCA2 showed a marked decrease earlier, i.e., at 20 weeks of age, coinciding with dropout and with histologically evident OA (5). Moreover, PCA2 patterns differed significantly between STR/Ort and CBA mice (P < 0.0001), indicating that PCA2 gait measures might correlate with OA progression. Paw area had the highest loading for PCA2 (Figure 1A), showed similar aging-related patterns in both strains of mice, and decreased at 28 weeks in CBA mice and 20 weeks in STR/Ort mice (Figure 3B). The symmetry index for paw area showed variation with age in CBA mice, with higher asymmetry compared to STR/Ort mice. The pattern of change over time in STR/Ort mice demonstrated increased asymmetry in paw area only from 28 weeks of age, when OA was progressing (Figure 3C).
Figure 3.
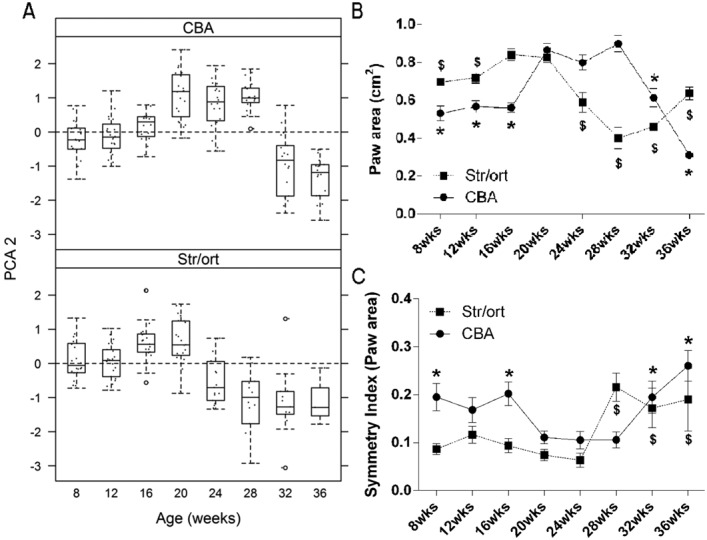
Longitudinal variation in the second principal component analysis (PCA2) and its individual component (paw area) reveals changes with aging in CBA and STR/Ort mice. A, Changes in PCA2 in CBA mice and STR/Ort mice between 8 and 36 weeks of age. Significant differences were noted between strains and over time. Data are shown as box plots. Each box represents the 25th to 75th percentiles. Lines inside the boxes represent the median. Dashed lines outside the boxes represent the 10th and the 90th percentiles. Dots represent individual mice. Circles indicate outliers. B, Paw area (mean of the left limbs and right limbs of each animal) in CBA and STR/Ort mice. In CBA mice paw area increased between 16 and 20 weeks of age and decreased from 28 weeks, whereas in STR/Ort mice paw area decreased from 20 weeks of age. ∗ = statistically significant difference from 20-week value in CBA mice (P = 0.000 at 8 weeks, 12 weeks, 16 weeks, 32 weeks, and 36 weeks); $ = statistically significant difference from 20-week value in STR/Ort mice (P = 0.001 at 8 weeks, P = 0.003 at 12 weeks, P = 0.000 at 24 weeks, 28 weeks, and 32 weeks, and P = 0.017 at 36 weeks). C, Symmetry index for paw area in CBA and STR/Ort mice. In STR/Ort mice, the symmetry index was similar between 8 and 24 weeks of age and showed significant increases in asymmetry from 28 weeks of age, whereas CBA mice exhibited diminished asymmetry between 20 and 28 weeks of age. ∗ = statistically significant difference from 20-week value in CBA mice (P = 0.014 at 8 weeks, P = 0.008 at 16 weeks, P = 0.016 at 32 weeks, and P = 0.000 at 36 weeks); $ = statistically significant difference from 20-week value in STR/Ort mice (P = 0.000 at 28 weeks, P = 0.001 at 32 weeks, and P = 0.019 at 36 weeks). Values in B and C are the mean ± SEM. For CBA mice, n = 20 at all time points; for STR/Ort mice, n = 22 at weeks 8, 12, and 16, n = 21 at week 20, n = 17 at week 24, n = 14 at week 28, n = 13 at week 32, and n = 3 at week 36.
Gait changes in osteoarthritic STR/Ort mice are not due to pain
To determine whether gait differences could be attributed to OA pain, gait was monitored in 34-week-old STR/Ort mice that had been provided with pain relief by treatment with carprofen for 3 days. This palliative treatment (24) did not modify any gait measurements or treadmill noncompliance, suggesting that gait changes in the STR/Ort mice were not due to pain. To examine whether OA development in these mice was associated with pain, sensitivity measures were obtained in mice between 16 and 36 weeks of age (study 5), by which age STR/Ort mice normally develop significant OA pathology. In these analyses STR/Ort mice did not display any significant changes in response to paw pressure, cold sensitivity, mechanical allodynia (von Frey filaments), or pain-related vocalizations, with all results similar to those obtained in CBA mice (Figure 4). To assess whether OA-related pain may be inhibited by naturally occurring opioids in STR/Ort mice (20,25), naloxone was administered. This treatment did not result in modification of any pain sensitivity measures in STR/Ort mice (Table2).
Figure 4.
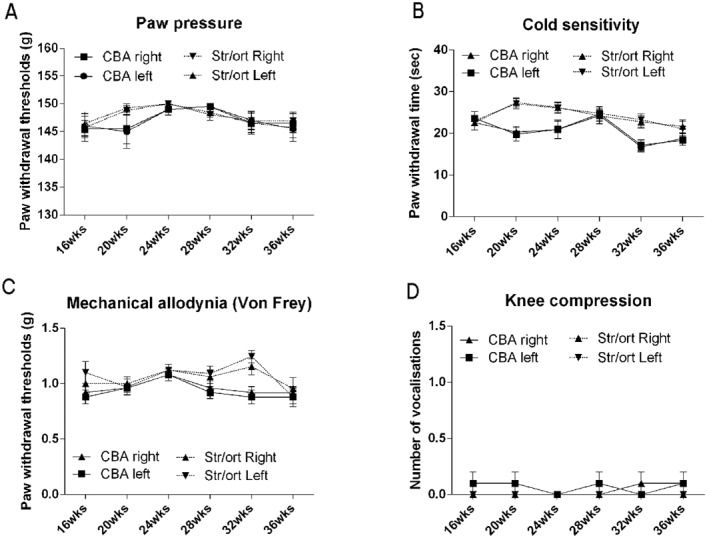
Pain behavior in STR/Ort and CBA mice does not show significant variation with age. Paw pressure (A), cold sensitivity (B), mechanical allodynia (von Frey filaments) (C), and number of vocalizations following knee compression (D) were each measured once a month in the left and right hind limbs of CBA and STR/Ort mice, from 16 weeks to 36 weeks of age. No significant changes with time were identified in either strain. (In contrast, typical values for hypersensitivity in a study of surgically induced osteoarthritis in mice [20] were as follows: paw pressure 60–70g, cold sensitivity 5–7 seconds, mechanical allodynia [von Frey filaments] 0.1–0.2g, and vocalizations 2.5–3.) Values are the mean ± SEM.
Table 2.
Pain behavior in CBA and STR/Ort mice after treatment with the opioid antagonist naloxone*
| CBA mice |
STR/Ort mice |
|||
|---|---|---|---|---|
| Left hind limb | Right hind limb | Left hind limb | Right hind limb | |
| Paw pressure (paw withdrawal threshold), g | ||||
| Predose (vehicle) | 147 ± 2 | 148 ± 1 | 146 ± 3 | 146 ± 3 |
| 1 hour posttreatment vehicle) | 143 ± 5 | 143 ± 5 | 145 ± 3 | 147 ± 2 |
| Predose (naloxone) | 144 ± 2 | 145 ± 3 | 147 ± 1 | 149 ± 1 |
| 1 hour posttreatment (naloxone) | 146 ± 3 | 146 ± 3 | 137 ± 3 | 140 ± 2 |
| Cold sensitivity (paw withdrawal time), seconds | ||||
| Predose (vehicle) | 19 ± 1 | 19 ± 1 | 21 ± 3 | 22 ± 3 |
| 1 hour posttreatment (vehicle) | 19 ± 1 | 20 ± 1 | 21 ± 2 | 21 ± 2 |
| Predose (naloxone) | 18 ± 2 | 18 ± 2 | 21 ± 2 | 22 ± 2 |
| 1 hour posttreatment (naloxone) | 20 ± 2 | 20 ± 2 | 19 ± 2 | 20 ± 3 |
| Vocalizations, number | ||||
| Predose (vehicle) | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| 1 hour posttreatment (vehicle) | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| Predose (naloxone) | 0 ± 0 | 0.2 ± 0.2 | 0 ± 0 | 0 ± 0 |
| 1 hour posttreatment (naloxone) | 0.6 ± 0.2 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| Mechanical allodynia (paw withdrawal threshold), g | ||||
| Predose (vehicle) | 0.8 ± 0.1 | 0.9 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.1 |
| 1 hour posttreatment (vehicle) | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.7 ± 0.1 | 0.9 ± 0.1 |
| Predose (naloxone) | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.7 ± 0.1 | 0.8 ± 0.1 |
| 1 hour posttreatment (naloxone) | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.7 ± 0.1 | 0.8 ± 0.1 |
Paw pressure, cold sensitivity, mechanical allodynia (von Frey filaments), and number of vocalizations following knee compression were measured before and 1 hour after intraperitoneal treatment with either control vehicle (n = 10 CBA mice and 13 STR/Ort mice) or naloxone (n = 10 CBA mice and 13 STR/Ort mice). Analysis by unpaired t-test revealed that no significant changes were induced by the treatments in mice of either strain. Values are the mean ± SEM.
Effects of CFA-induced joint inflammation were also examined to determine if absence of pain behaviors in STR/Ort mice was due to a general insensitivity. Three 40–43-week-old STR/Ort mice injected with CFA developed the expected range of pain-related behaviors, with significant increases in sensitivity to mechanical stimulation (from a mean ± SEM of 0.6 ± 0g to 0.24 ± 0.08g on von Frey filament testing and 155.00 ± 5.77g to 96.67 ± 3.33g on paw pressure testing), knee compression (from 0 ± 0 vocalizations to 1 ± 0.58 vocalizations), and cold (from 23.30 ± 3.37 seconds to 15.93 ± 1.94 seconds). These data indicate that the absence of pain behaviors in STR/Ort mice is likely not due to general pain insensitivity and that gait changes occurring concomitantly with OA development in STR/Ort mice may not be attributable to joint pain.
DISCUSSION
In this study we used video-based analyses to identify gait changes in mice with naturally occurring OA. We discovered links between specific gait modifications and OA severity, with paw area identified as being potentially predictive. By examining pain-associated behaviors, we also demonstrated that gait changes in STR/Ort mice with OA are not accompanied by measurable joint pain, indicating that hind limb paw area measurement may be a useful, noninvasive monitoring tool that likely reflects structural, rather than pain-related, changes in the joint. In addition, we found that treadmill task noncompliance may serve as an indicator of OA in these mice.
STR/Ort mice have predictable disease development, with initial articular cartilage lesions occurring at age 18–20 weeks (6) and progression to severe OA by 12 months (5). These mice develop characteristics resembling those seen in human OA, including osteophytes, subchondral bone sclerosis, and synovial hyperplasia (1,7,9). CBA mice were used as controls for 2 reasons: they are the closest commercially available genetic relative (used in initial cross to generate STR/Ort mice [17,18]), and are very resistant to age-related spontaneous OA (5). Inclusion of CBA mice (as in study 4) allows the confounding effects of aging to be limited.
Gait analysis has been attempted in only one previously reported study of STR/Ort mice (26). Use of ink pawprints revealed reduced variance in gait in aspartame-fed mice in which histologically defined OA was delayed. The data presented herein cannot be compared with these findings; however, we have noted increased variability in swing time in STR/Ort mice compared to CBA mice (Poulet B, et al: unpublished observations). Inherent strain-related differences were centered around stride and stance, as well as around propel and brake times, which are components of stance times. Stride frequency and length and swing time, a component of stride, were significantly different between strains at all ages, with STR/Ort showing larger steps and longer components of stride than CBA mice. Differences in body dimension in STR/Ort mice, such as weight and tibia length (data not shown), may be a factor in inherent gait differences that lead to OA in these mice.
Gait has previously been analyzed in models of OA and inflammatory arthritis. Analysis of type IX collagen–deficient mice, which develop OA spontaneously, revealed reduced stride length and increased stance times compared to wild-type mice (27). Induction of OA in transforming growth factor β1–injected mice by treadmill running also led to increased stance times and decreased swing time (15). Paw area was decreased in rodents with carrageenan-induced rheumatoid arthritis (28), but was increased after development of swelling and inflammation in collagen-induced arthritis (29). Contralateral limbs were not evaluated in either of those studies. It is evident that decreased paw area suggests greater weight focus to a smaller foot area, consistent with the protection of specific joint locations from weight bearing.
We found that paw area was correlated with OA severity in STR/Ort mice, with a statistically significant correlation observed only in comparisons between mice with severe OA (grades 5–6) and mice with mild OA (grades 0–4). In these mice, OA severity was linked closely with modified paw area, suggesting a strong association between gait impairment and disease severity. Longitudinal analysis, however, showed early decreases in paw area. These decreases may have multiple origins, including the following: 1) less weight being passed through that limb and more through the contralateral, presumably less painful, limb might result in decreased paw splaying; 2) muscles in a painful (or osteoarthritic) limb may be contracting in a manner that reduces pain, pulling the paw together (digital flexors) to reduce paw area; or 3) joint angles may be changed to deliver a more upright limb, reducing joint moments and, hence, muscle forces.
Stratifying individual limb kinematics by OA scores in individual limbs may provide better insight into OA-dependent kinematic changes. Such stratification would, however, be biased to only the OA-affected limb. Our use of an unbiased approach, also examining gait in both limbs, allows for consideration of any changes in the contralateral limb. This is relevant in this OA model, in which disease can affect left and right limbs independently with age, unlike other models of acute arthritis pain (29), in which the diseased or affected limb is known. We used a symmetry index as a way of measuring gait asymmetries in hind limbs, revealing asymmetries in paw area with aging in both osteoarthritic STR/Ort and control CBA mice. Further studies are under way to identify asymmetries between all 4 limbs in more detail and to investigate their associations with OA severity.
Gait changes in models of chronic inflammation are associated mainly with increased pain sensitivity (14,28,29). This contrasts with the present findings of age-related gait changes, not associated with pain-related behaviors, in CBA and STR/Ort mice. Our results suggest that age-related modifications in gait are instead due to structural changes. Similar conclusions have been drawn from studies of human OA (30–33), with only comparatively few studies having been conducted in murine species (16). Healthy joint aging is known to involve tendon stiffening (34), which may modify gait by restricting joint movement and full heel–toe strike. Decreased paw area in STR/Ort mice coincides with OA onset at age 20 weeks, and later articular changes, including osteophytes (8), ligament weakness, and chondro-osseous changes (5,35,36), may in turn contribute to gait modification in these mice.
As the etiology of OA in this model remains to be defined, there are factors that may confound interpretation of our data, including soft tissue calcification, patellar subluxation, ankle deformity, and obesity (37–39). Similar caveats apply to the possibility that differences in limb size and weight gain trajectories in CBA and STR/Ort mice may affect gait. It is possible, therefore, that the gait patterns we have monitored may not reflect functional biomechanical changes that occur during OA development, and this remains a significant limitation of our study. The changes in gait may reflect normal aging processes, a consequence of OA-dependent neuromuscular change, or some as-yet-unidentified modification in these mice (40,41). Our study revealed that CBA mice readily complied with treadmill running tasks at all ages, whereas STR/Ort mouse dropout coincided temporally with histologically detectable OA at age 20 weeks. We propose that dropout counts may provide a noninvasive measure of OA in appropriately sized STR/Ort mouse groups. We did not ascertain whether dropout is linked to a specific OA feature, and future work will be undertaken to define whether a specific physical impairment underlies this phenomenon.
A significant and somewhat surprising result of this study was the lack of pain sensitivity in osteoarthritic STR/Ort mice. Responses to mechanical allodynia or paw pressure, cold sensitivity, and joint compression–related vocalization did not differ between these mice and controls, and no modification in their gait was observed after carprofen or opioid antagonist treatment. These intriguing observations should be placed into context with OA in humans, with pain not always experienced despite radiographic diagnosis (42–44). Indeed, ∼60% of patients with OA diagnosed by a physician or confirmed radiographically failed to report knee pain (43). This is likely to contribute to late diagnosis and limitation of protective measures, and to exacerbate known propensities toward OA in the contralateral limb. Our data identify STR/Ort mice as a model of asymptomatic OA and highlight the novel and exciting putative utility of this model in identifying markers and processes of joint degeneration without the complicating factor of pain.
Long-term changes in gait may not be reversible with short-term analgesia. Indeed, it is possible that initial gait adaptation could be due to pain, but repeating this adaptation and avoiding pain during locomotion may lead the musculoskeletal system to remodel over time and program the abnormality into the gait sequence. The subsequent blocking of pain mechanisms long after a gait modification has been established, as tested with carprofen treatment in this study, may not immediately modify the gait abnormality. Even in the clinical setting, physical therapy or training may be needed to reset the gait sequence and this is, in part, a large motivation for physical therapy and exercise as a treatment for OA. In addition, although no pain in STR/Ort mice before or after the onset of OA was detected using our methods, we can only suggest that abnormalities in gait in these mice are not due to pain. In addition, the reaction to pain can be complex and not always intuitive (42,43) and does not always result in a simple unloading of the affected body part. Direct measurement of limb forces would help in exploring these effects but such measurements, while common in studies of larger animals (45), are challenging in animals the size of a transgenic mouse.
Noninvasive murine gait measurement is technically challenging, with most studies using “footprint” analysis (26). DigiGait involves video-recording and benefits from automatic computing with minimal operator error, and from treadmill use with adjustable running speed that allows adjustments that can reveal gait changes only detectable at specific speeds. Another advantage is the high reproducibility of data recorded upon repeat treadmill testing in the same mouse. Measurement of brake and propel times, however, has not been validated by force plate analysis, and thus these data must be considered with caution.
The present findings indicate that noninvasive changes in gait, unrelated to classic joint pain measures, are useful in monitoring OA in STR/Ort mice. Our study identifies modifications in paw area as being a particularly useful parameter and treadmill noncompliance as a potential means of monitoring development of nonpainful, structural OA in these mice. We speculate that this will facilitate future longitudinal noninvasive assessments of new therapies to help slow the progression of OA. It may also facilitate reduction and refinement of animal use by partially replacing histologic methods for OA grading.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Pitsillides had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Poulet, de Souza, Knights, Wilson, Bevan, Pitsillides.
Acquisition of data. Poulet, de Souza, Knights, Gentry, Wilson.
Analysis and interpretation of data. Poulet, de Souza, Knights, Gentry, Bevan, Chang, Pitsillides.
Acknowledgments
We are grateful to Dr. J. Morton (University of Cambridge, Cambridge, UK) for providing us with the DigiGait system for gait analysis.
REFERENCES
- 1.Mason RM, Chambers MG, Flannelly J, Gaffen JD, Dudhia J, Bayliss MT. The STR/ort mouse and its use as a model of osteoarthritis. Osteoarthritis Cartilage. 2001;9:85–91. doi: 10.1053/joca.2000.0363. [DOI] [PubMed] [Google Scholar]
- 2.Glasson SS, Askew R, Sheppard B, Carito B, Blanchet T, Ma HL, et al. Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature. 2005;434:644–8. doi: 10.1038/nature03369. [DOI] [PubMed] [Google Scholar]
- 3.Ma HL, Blanchet TJ, Peluso D, Hopkins B, Morris EA, Glasson SS. Osteoarthritis severity is sex dependent in a surgical mouse model. Osteoarthritis Cartilage. 2007;15:695–700. doi: 10.1016/j.joca.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Majumdar MK, Askew R, Schelling S, Stedman N, Blanchet T, Hopkins B, et al. Double-knockout of ADAMTS-4 and ADAMTS-5 in mice results in physiologically normal animals and prevents the progression of osteoarthritis. Arthritis Rheum. 2007;56:3670–4. doi: 10.1002/art.23027. [DOI] [PubMed] [Google Scholar]
- 5.Walton M. Degenerative joint disease in the mouse knee: histological observations. J Pathol. 1977;123:109–22. doi: 10.1002/path.1711230207. [DOI] [PubMed] [Google Scholar]
- 6.Poulet B, Ulici V, Stone TC, Pead M, Gburcik V, Constantinou E, et al. Time-series transcriptional profiling yields new perspectives on susceptibility to murine osteoarthritis. Arthritis Rheum. 2012;64:3256–66. doi: 10.1002/art.34572. [DOI] [PubMed] [Google Scholar]
- 7.Chambers MG, Cox L, Chong L, Suri N, Cover P, Bayliss MT, et al. Matrix metalloproteinases and aggrecanases cleave aggrecan in different zones of normal cartilage but colocalize in the development of osteoarthritic lesions in STR/ort mice. Arthritis Rheum. 2001;44:1455–65. doi: 10.1002/1529-0131(200106)44:6<1455::AID-ART241>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 8.Blaney Davidson EN, Vitters EL, van Beuningen HM, van de Loo FA, van den Berg WB, van der Kraan PM. Resemblance of osteophytes in experimental osteoarthritis to transforming growth factor β–induced osteophytes: limited role of bone morphogenetic protein in early osteoarthritic osteophyte formation. Arthritis Rheum. 2007;56:4065–73. doi: 10.1002/art.23034. [DOI] [PubMed] [Google Scholar]
- 9.Walton M, Elves MW. Bone thickening in osteoarthrosis: observations of an osteoarthrosis-prone strain of mouse. Acta Orthop Scand. 1979;50:501–6. doi: 10.3109/17453677908989795. [DOI] [PubMed] [Google Scholar]
- 10.Astephen Wilson JL, Deluzio KJ, Dunbar MJ, Caldwell GE, Hubley-Kozey CL. The association between knee joint biomechanics and neuromuscular control and moderate knee osteoarthritis radiographic and pain severity. Osteoarthritis Cartilage. 2011;19:186–93. doi: 10.1016/j.joca.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 11.Lo GH, Harvey WF, McAlindon TE. Associations of varus thrust and alignment with pain in knee osteoarthritis. Arthritis Rheum. 2012;64:2252–9. doi: 10.1002/art.34422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang A, Hurwitz D, Dunlop D, Song J, Cahue S, Hayes K, et al. The relationship between toe-out angle during gait and progression of medial tibiofemoral osteoarthritis. Ann Rheum Dis. 2007;66:1271–5. doi: 10.1136/ard.2006.062927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mundermann A, Dyrby CO, Andriacchi TP. Secondary gait changes in patients with medial compartment knee osteoarthritis: increased load at the ankle, knee, and hip during walking. Arthritis Rheum. 2005;52:2835–44. doi: 10.1002/art.21262. [DOI] [PubMed] [Google Scholar]
- 14.Vincelette J, Xu Y, Zhang LN, Schaefer CJ, Vergona R, Sullivan ME, et al. Gait analysis in a murine model of collagen-induced arthritis. Arthritis Res Ther. 2007;9:R123. doi: 10.1186/ar2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Plaas A, Li J, Riesco J, Das R, Sandy JD, Harrison A. Intraarticular injection of hyaluronan prevents cartilage erosion, periarticular fibrosis and mechanical allodynia and normalizes stance time in murine knee osteoarthritis. Arthritis Res Ther. 2011;13:R46. doi: 10.1186/ar3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allen KD, Griffin TM, Rodriguiz RM, Wetsel WC, Kraus VB, Huebner JL, et al. Decreased physical function and increased pain sensitivity in mice deficient for type IX collagen. Arthritis Rheum. 2009;60:2684–93. doi: 10.1002/art.24783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strong LC. Genetic nature of the constitutional states of cancer susceptibility and resistance in mice and men. Yale J Biol Med. 1945;73:265–72. [PubMed] [Google Scholar]
- 18.Silverstein E, Sokoloff L, Mickelsen O, Jay GE. Primary polydipsia and hydronephrosis in an inbred strain of mice. Am J Pathol. 1961;38:143–59. [PMC free article] [PubMed] [Google Scholar]
- 19.Glasson SS, Chambers MG, van den Berg WB, Little CB. The OARSI histopathology initiative—recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthritis Cartilage. 2010;18(Suppl 3):S17–23. doi: 10.1016/j.joca.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 20.Knights CB, Gentry C, Bevan S. Partial medial meniscectomy produces osteoarthritis pain-related behaviour in female C57BL/6 mice. Pain. 2012;153:281–92. doi: 10.1016/j.pain.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 21.Fernihough J, Gentry C, Malcangio M, Fox A, Rediske J, Pellas T, et al. Pain related behaviour in two models of osteoarthritis in the rat knee. Pain. 2004;112:83–93. doi: 10.1016/j.pain.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 22.Randall LO, Selitto JJ. A method for measurement of analgesic activity on inflamed tissue. Arch Int Pharmacodyn Ther. 1957;111:409–19. [PubMed] [Google Scholar]
- 23.Gentry C, Stoakley N, Andersson DA, Bevan S. The roles of iPLA2, TRPM8 and TRPA1 in chemically induced cold hypersensitivity. Mol Pain. 2010;6:4. doi: 10.1186/1744-8069-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lipscomb VJ, AliAbadi FS, Lees P, Pead MJ, Muir P. Clinical efficacy and pharmacokinetics of carprofen in the treatment of dogs with osteoarthritis. Vet Rec. 2002;150:684–9. doi: 10.1136/vr.150.22.684. [DOI] [PubMed] [Google Scholar]
- 25.Inglis JJ, McNamee KE, Chia SL, Essex D, Feldmann M, Williams RO, et al. Regulation of pain sensitivity in experimental osteoarthritis by the endogenous peripheral opioid system. Arthritis Rheum. 2008;58:3110–9. doi: 10.1002/art.23870. [DOI] [PubMed] [Google Scholar]
- 26.Manion CV, Hochgeschwender U, Edmundson AB, Hugli TE, Gabaglia CR. Dietary aspartyl-phenylalanine-1-methyl ester delays osteoarthritis and prevents associated bone loss in STR/ORT mice. Rheumatology (Oxford) 2011;50:1244–9. doi: 10.1093/rheumatology/ker089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Costello KE, Guilak F, Setton LA, Griffin TM. Locomotor activity and gait in aged mice deficient for type IX collagen. J Appl Physiol (1985) 2010;109:211–8. doi: 10.1152/japplphysiol.00056.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heilborn U, Berge OG, Arborelius L, Brodin E. Spontaneous nociceptive behaviour in female mice with Freund's complete adjuvant- and carrageenan-induced monoarthritis. Brain Res. 2007;1143:143–9. doi: 10.1016/j.brainres.2007.01.054. [DOI] [PubMed] [Google Scholar]
- 29.Berryman ER, Harris RL, Moalli M, Bagi CM. Digigait quantitation of gait dynamics in rat rheumatoid arthritis model. J Musculoskelet Neuronal Interact. 2009;9:89–98. [PubMed] [Google Scholar]
- 30.Wang TM, Yen HC, Lu TW, Chen HL, Chang CF, Liu YH, et al. Bilateral knee osteoarthritis does not affect inter-joint coordination in older adults with gait deviations during obstacle-crossing. J Biomech. 2009;42:2349–56. doi: 10.1016/j.jbiomech.2009.06.029. [DOI] [PubMed] [Google Scholar]
- 31.Federolf PA, Boyer KA, Andriacchi TP. Application of principal component analysis in clinical gait research: identification of systematic differences between healthy and medial knee-osteoarthritic gait. J Biomech. 2013;46:2173–8. doi: 10.1016/j.jbiomech.2013.06.032. [DOI] [PubMed] [Google Scholar]
- 32.Esrafilian A, Karimi MT, Amiri P, Fatoye F. Performance of subjects with knee osteoarthritis during walking: differential parameters. Rheumatol Int. 2013;33:1753–61. doi: 10.1007/s00296-012-2639-2. [DOI] [PubMed] [Google Scholar]
- 33.Butler RJ, Barrios JA, Royer T, Davis IS. Frontal-plane gait mechanics in people with medial knee osteoarthritis are different from those in people with lateral knee osteoarthritis. Phys Ther. 2011;91:1235–43. doi: 10.2522/ptj.20100324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dudhia J, Scott CM, Draper ER, Heinegard D, Pitsillides AA, Smith RK. Aging enhances a mechanically-induced reduction in tendon strength by an active process involving matrix metalloproteinase activity. Aging Cell. 2007;6:547–56. doi: 10.1111/j.1474-9726.2007.00307.x. [DOI] [PubMed] [Google Scholar]
- 35.Walton M. Degenerative joint disease in the mouse knee: radiological and morphological observations. J Pathol. 1977;123:97–107. doi: 10.1002/path.1711230206. [DOI] [PubMed] [Google Scholar]
- 36.Anderson-MacKenzie JM, Billingham ME, Bailey AJ. Collagen remodeling in the anterior cruciate ligament associated with developing spontaneous murine osteoarthritis. Biochem Biophys Res Commun. 1999;258:763–7. doi: 10.1006/bbrc.1999.0713. [DOI] [PubMed] [Google Scholar]
- 37.Walton M. Patella displacement and osteoarthrosis of the knee joint in mice. J Pathol. 1979;127:165–72. doi: 10.1002/path.1711270402. [DOI] [PubMed] [Google Scholar]
- 38.Walton M. Obesity as an aetiological factor in the development of osteoarthrosis. Gerontology. 1979;25:36–41. doi: 10.1159/000212318. [DOI] [PubMed] [Google Scholar]
- 39.Walton M. A spontaneous ankle deformity in an inbred strain of mouse. J Pathol. 1978;124:189–94. doi: 10.1002/path.1711240403. [DOI] [PubMed] [Google Scholar]
- 40.Rudolph KS, Schmitt LC, Lewek MD. Age-related changes in strength, joint laxity, and walking patterns: are they related to knee osteoarthritis? Phys Ther. 2007;87:1422–32. doi: 10.2522/ptj.20060137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Messier SP. Osteoarthritis of the knee and associated factors of age and obesity: effects on gait. Med Sci Sports Exerc. 1994;26:1446–52. [PubMed] [Google Scholar]
- 42.Bedson J, Croft PR. The discordance between clinical and radiographic knee osteoarthritis: a systematic search and summary of the literature. BMC Musculoskelet Disord. 2008;9:116. doi: 10.1186/1471-2474-9-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hannan MT, Felson DT, Pincus T. Analysis of the discordance between radiographic changes and knee pain in osteoarthritis of the knee. J Rheumatol. 2000;27:1513–7. [PubMed] [Google Scholar]
- 44.Schiphof D, Kerkhof HJ, Damen J, de Klerk BM, Hofman A, Koes BW, et al. Factors for pain in patients with different grades of knee osteoarthritis. Arthritis Care Res (Hoboken) 2013;65:695–702. doi: 10.1002/acr.21886. [DOI] [PubMed] [Google Scholar]
- 45.Williams GE, Silverman BW, Wilson AM, Goodship AE. Disease-specific changes in equine ground reaction force data documented by use of principal component analysis. Am J Vet Res. 1999;60:549–55. [PubMed] [Google Scholar]


