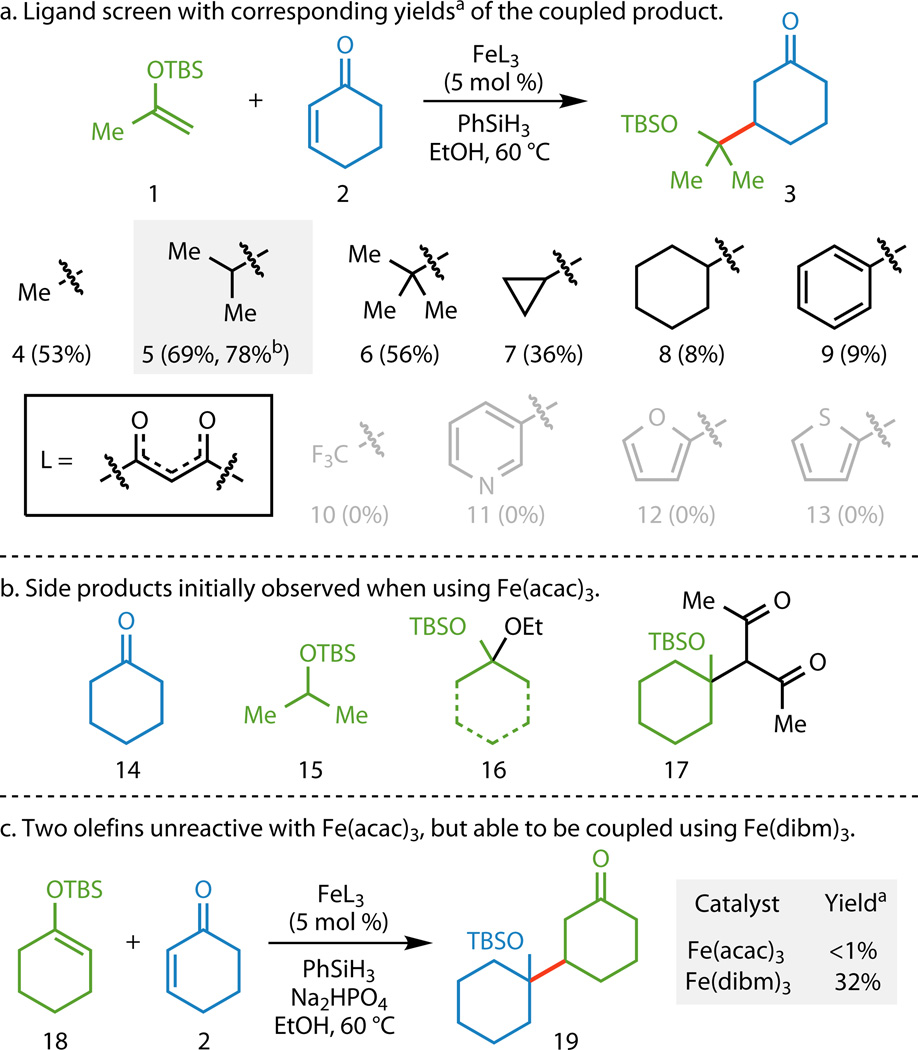
Figure 2. Functionalized olefin cross-coupling optimization studies.
a, Altering the ligands on the Fe center had the greatest influence on the outcome of the reaction with Fe(dibm)3 (5) giving the highest yields. The addition of Na2HPO4 further increased the yield. b, Side products that were observed when Fe(acac)3 (4) was used as the catalyst. The formation of compounds 16 and 17 could be attributed to the Lewis acidity of 4. The use of 5 as the catalyst reduced the formation of compounds 16 and 17. c, An example where the use of 5 instead of 4 was essential in obtaining the desired functionalized olefin cross-coupling reactivity. aYields based on GC/MS analysis using 1,3,5-trimethoxybenzene as an internal standard. bUsing 1 equiv Na2HPO4 as an additive. TBS, tert-butyldimethylsilyl; L, ligand; acac, acetylacetonate; dibm, diisobutyrylmethane; GC/MS, gas chromatography/mass spectrometry.