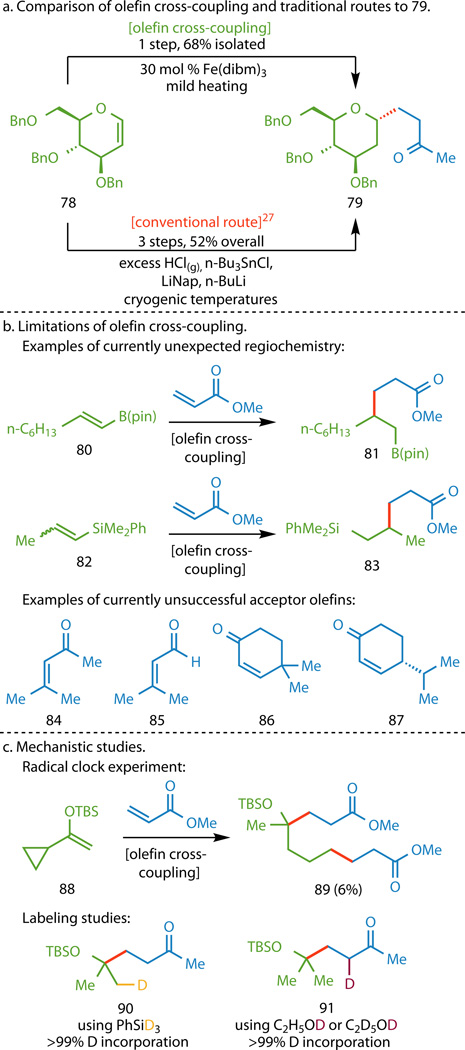
Figure 4. Additional functionalized olefin cross-coupling studies.
a, Functionalized olefin cross-coupling offers a direct route to glucal derivative 79 that circumvents the use of harsh reagents, superstoichiometric organometallic reagents, and cryogenic temperatures in traditional approaches. b, The use of certain 1,2-disubstituted donor olefins (80 and 82) gave adducts where the C–C bond formed distal instead of adjacent to the heteroatom (81 and 83). Additionally, the use of acceptors with excessive aliphatic substitution (84–87) gave trace or no product. c, The use of vinyl cyclopropane 88 resulted in the isolation of 89, where the fragmentation of the cyclopropane ring supports the formation of a radical adjacent to the heteroatom in the donor. Isolation of compounds 90 and 91 from deuterium labeling studies further support the mechanism depicted in Figure 1B.