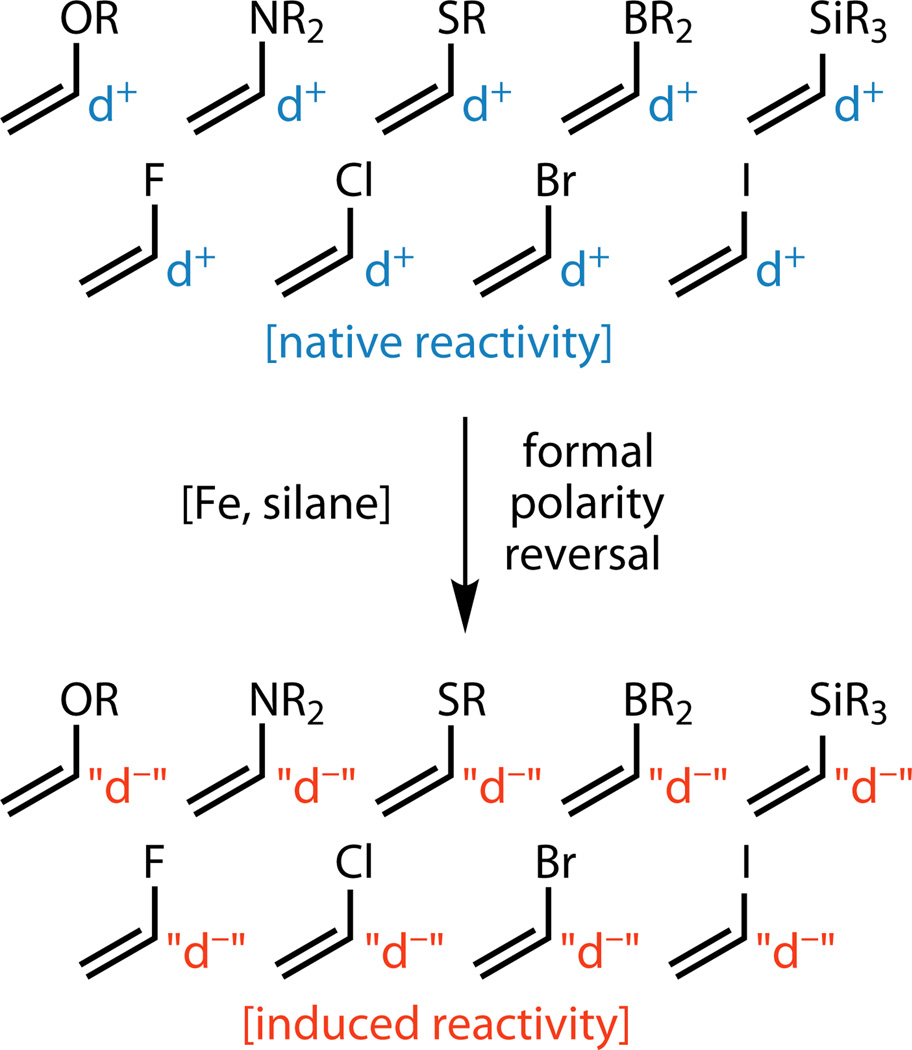
Figure 5. Functionalized olefin cross-coupling reverses conventional reactivity expectations.
The substrates employed as donors in this study typically are electrophilic at the position adjacent to the heteroatom. Functionalized olefin cross-coupling reverses this reactivity via the intermediacy of radicals, resulting in those same positions bearing nucleophilic properties.