Abstract
The transgenic adenocarcinoma of the mouse prostate (TRAMP) model is well established and offers several advantages for the study of chemopreventive agents, including its well-defined course of disease progression and high incidence of poorly differentiated carcinomas within a relatively short length of time. However, there is no consensus on the grading of prostatic lesions in these mice. In particular, agreement is lacking on the criteria for differentiating prostatic intraepithelial neoplasia (PIN) from well-differentiated adenocarcinoma, specifically as it relates to evidence of invasion. This differentiation is critical for evaluating the effects of putative chemopreventive agents on progression to neoplasia. Moreover, only one of the published grading schemes assigns numerical grades to prostatic lesions, which facilitate statistical analysis. Here, we review five currently available grading schemes and propose a refined scheme that provides a useful definition of invasion for the differentiation of PIN from well-differentiated adenocarcinoma and includes a numerical scoring system that accounts for both the most severe and most common histopathological lesions in each of the lobes of the prostate and their distributions. We expect that researchers will find this refined grading scheme to be useful for chemoprevention studies in TRAMP mice.
Keywords: TRAMP, prostate, prostate cancer, lesion grades, chemoprevention
Introduction
In the transgenic adenocarcinoma of the mouse prostate (TRAMP) model, the SV40 large and small T antigens are expressed under the androgen-dependent control of the rat probasin promoter in the prostatic epithelium. Expression of the transgene results in inhibition of p53 and Rb tumor suppressor activities (Gingrich et al. 1999; Gingrich et al. 1996; Greenberg et al. 1994, 1995; Greenberg 1996). Two lines of TRAMP mice exist: the C57BL/6 parent line and the first-generation offspring of the parent line crossed to FvB mice (C57BL/6 TRAMP × FvB). In the literature, both lines are referred to as TRAMP mice. The two lines exhibit similar progression of prostate lesions, with the lesions of C57BL/6 TRAMP × FvB mice progressing at a slightly more rapid rate (Gingrich et al. 1999).
Male TRAMP mice reproducibly develop poorly differentiated prostate carcinomas. The prostates of these mice progress through different preneoplastic and neoplastic lesions, similar to what occurs in man (Kaplan-Lefko et al. 2003; Gingrich et al. 1999; Greenberg et al. 1995; Gingrich et al. 1996). Between 6 and 12 wk of age, the prostatic epithelial cells develop varying degrees of hyperplasia or prostatic intraepithelial neoplasia (PIN). By approximately 18 wk of age, mice develop well-differentiated prostatic adenocarcinomas. Between the ages of 24 and 30 wk, almost 100%of the TRAMP mice will have poorly differentiated carcinomas, with metastasis to the iliac lymph nodes and lungs most commonly (Kaplan-Lefko et al. 2003; Gingrich et al. 1996; Gingrich et al. 1999; Hurwitz et al. 2001; Greenberg 1996). Because of tumor size and metastatic burden, C57BL/6 TRAMP × FvB mice rarely live beyond 33 wk of age, while C57BL/6 TRAMP mice are frequently able to live for up to 36 to 40 wk, with occasional mice surviving up to 52 wk of age (Gingrich et al. 1999).
In addition to progressing through the various preneoplastic and neoplastic stages similar to those observed in men, tumorigenesis in the TRAMP model has other similarities to human prostate carcinogenesis. As in men, prostate cancer of TRAMP mice progresses from an initial androgen-dependent stage to a castration-resistant stage. Castration at 12 wk of age results in approximately 80% of C57BL/6 TRAMP × FvB mice developing hormone-insensitive, poorly differentiated carcinomas by 24 wk of age, which metastasize at a higher frequency than those in intact mice (Gingrich et al. 1997; Kaplan-Lefko et al. 2003). Androgen receptor immunostaining is variable, and may even be absent, in moderately differentiated or poorly differentiated carcinomas (Kaplan-Lefko et al. 2003). Similar to immunohistochemical changes observed in human prostatic carcinomas, decreased cytokeratin 8 and E-cadherin immunostaining are observed in poorly differentiated carcinomas of TRAMP mice compared with PIN and well-differentiated adenocarcinomas. The changes in tumor cell immunoreactivity suggest that an epithelial-to-mesenchymal transition is occurring with loss of tumor differentiation (Kaplan-Lefko et al. 2003).
Despite the similarities in the progression of prostate lesions in men and TRAMP mice, controversy exists regarding the applicability of the TRAMP model for the study of human prostate tumorigenesis. The main point of contention is the existence of the neuroendocrine phenotype. Poorly differentiated carcinomas in the TRAMP model frequently exhibit a neuroendocrine phenotype with positive immunostaining for neuroendocrine markers, such as synaptophysin (Chiaverotti et al. 2008; Kaplan-Lefko et al. 2003). There are two opinions regarding the origin of these neuroendocrine cells. Since pre-neoplastic PIN lesions and well-differentiated adenocarcinomas rarely express neuroendocrine markers, it is speculated that the poorly differentiated carcinomas arise from cells undergoing epithelial-to-neuroendocrine transition with loss of differentiation (Kaplan-Lefko et al. 2003), analogous to what occurs in men (Bonkhoff 2001). The second opinion is that the poorly differentiated carcinomas arise from a neuroendocrine stem cell with no relation to the other lesions occurring in the prostates (Chiaverotti et al. 2008). In part because we have observed prostates in which poorly differentiated carcinomas coexist with preexisting PIN or well-differentiated adenocarcinoma, we support the first hypothesis, namely, that tumor cells that express neuroendocrine markers have undergone epithelial-to-neuroendocrine transition. In addition, since approximately 10% of human prostate carcinomas have a neuroendocrine phenotype in association with increased aggressive behavior (Bonkhoff 2001), we do not believe that the neuroendocrine phenotype in poorly differentiated carcinomas of TRAMP mice obviates its use as a model for prostate tumorigenesis in man. Rather, we believe that the TRAMP model with its progression from preneoplastic lesions to highly aggressive neoplasia has an advantage over other transgenic mouse models of prostate cancer.
The TRAMP model has proven useful to investigate the ability of drugs to prevent preneoplastic lesions (PIN) from progressing to aggressive neoplasia. Interventions that have successfully slowed the progression of lesions in TRAMP mice and reduced the incidence of poorly differentiated carcinomas include the histone deacetylase inhibitor OSU-HDAC42 (a.k.a. AR42; Arno Therapeutics, Parsippany, NJ; Sargeant et al. 2008), the nonsteroidal anti-inflammatory drug E-7869 (R-flurbiprofen; Wechter et al. 2000), and 20% dietary restriction (Suttie et al. 2003). An efficient and inclusive grading scheme for the evaluation of prostate lesions in TRAMP mice is an important component of such chemoprevention studies. Unlike human prostate pathology, in which low-grade and high-grade PIN are used to describe the preneoplastic lesions and the Gleason system is used to grade prostate cancer (Kumar, Abbas, and Fausto 2005), multiple grading schemes for the lesions in the prostates of TRAMP mice exist (Gingrich et al. 1999; Kaplan-Lefko et al. 2003; Shappell et al. 2004; Suttie et al. 2003; Chiaverotti et al. 2008; Hurwitz et al. 2001).
Review of Available Grading Schemes for TRAMP Mice
The first comprehensive grading scheme for the C57BL/6 TRAMP and C57BL/6 TRAMP × FvB mice was described by Gingrich et al. in 1999. In this scheme, lesions were described as low-grade PIN, high-grade PIN, well-differentiated adenocarcinoma, moderately differentiated adenocarcinoma, or poorly differentiated adenocarcinoma. Low-grade PIN was defined based on the appearance of elongated, instead of round, nuclei with condensed chromatin. The designation of high-grade PIN was made based on nuclear morphology and cell changes, including variation in nuclear shape, condensed chromatin, and mitotic figures. Instead of being oriented in a single layer, prostate epithelial cells stratify and form cribriform and papillary structures. Well-differentiated adenocarcinoma was diagnosed when epithelial cells invaded into the stroma surrounding glands, or, more frequently, when epithelial cells with rounded nuclei were present within the hypertrophied and hyperplastic fibromuscular stroma surrounding the glands. The rounded nuclei were reported to be frequently immunohistochemically positive for the T antigen, suggesting that these rounded nuclei belong to invading transformed epithelial cells. The diagnosis of moderately differentiated adenocarcinoma was made based on the neoplasm containing irregularly shaped glands, while poorly differentiated adenocarcinoma was diagnosed if no glandular elements remained and the tumor was composed of solid sheets of cells (Gingrich et al. 1999).
Since the introduction of the grading scheme by Gingrich et al. (1999), other investigators have refined the classification of lesions of TRAMP mice and proposed modified grading schemes. Four of these modified schemes are reviewed here. The grading scheme proposed by Kaplan-Lefko et al. in 2003 for C57BL/6 TRAMP × FvB mice classified the prostatic lesions in TRAMP mice as PIN, well-differentiated carcinoma, moderately differentiated carcinoma, poorly differentiated carcinoma, or phyllodes-like lesions. The single grade of PIN described by the authors was characterized by cells exhibiting stratification and papillary projections, cribriform patterns, and tufts. Nuclei were reported to be elongated with hyperchromatic chromatin, and cells had increased mitotic and apoptotic indices. As PIN progressed to well-differentiated carcinoma, invasion was frequently noted. In the well-differentiated carcinomas, there were increased small glandular structures with desmoplasia. The cells had round nuclei, with less hyperchromasia compared with nuclei of PIN-containing glands, and mitotic and apoptotic indices were increased. Moderately differentiated carcinoma was distinguished from well-differentiated carcinoma by the replacement of the neoplastic glands by solid sheets of cells, with remnants of glandular structures. In the poorly differentiated carcinomas, the tumors were composed of solid sheets of cells with high nuclear to cytoplasmic ratios, anisokaryosis, and marked pleomorphism. Phyllodes-like lesions were composed of hypercellular stroma that was tightly packed or loose and edematous and exhibited epithelial changes comparable with those of PIN or carcinoma. The phylloides-like lesions occurred at a higher frequency in C57BL/6 TRAMP mice than in C57BL/6 TRAMP × FvB mice (Kaplan-Lefko et al. 2003).
In 2003, Suttie et al. proposed a grading scheme for the prostates of C57BL/6 TRAMP mice that recommended evaluating each lobe of the prostate and assigning a grade from 1 to 6, based on the most severe lesion within the lobe. Grades 1 through 3 represented varying degrees of hyperplasia, grades 4 and 5 denoted adenomas, and grade 6 indicated adenocarcinoma. The distribution of the lesion within each individual lobe was estimated and described as either focal (fewer than two lesions), multifocal (three or more lesions with less than 30% of the lobe involved), or diffuse (greater than 30% of the lobe involved). The distribution and lesion grade were then combined to calculate a distribution-adjusted lesion grade ranging from 0 to 18 that could be used for statistical analysis. Prostate glands with grade 1 hyperplasia were defined as those lined by hyperbasophilic epithelial cells with frequent crowding but no stratification. Glands with grade 2 hyperplasia were differentiated from grade 1 by occasional stratification of epithelial cells and the formation of papilla or cribriform structures. In grade 3 hyperplasia, papilla and cribriform structures that extended into the lumen of the gland were observed. Grade 3 hyperplasia may have concurrent hyperplasia of the smooth muscle surrounding the dorsal and anterior lobes of the prostate. Grade 4 adenomas were characterized by glands filled with proliferating epithelium. Hyperplasia of the smooth muscle surrounding the anterior and dorsal lobes may be more severe in grade 4 adenomas compared with grade 3 hyperplasia. The grade 5 adenoma was distinguished from the grade 4 adenoma based on enlargement of the gland. Finally, the grade 6 adenocarcinoma exhibited poorly differentiated epithelial cells with local invasion through the capsule and/or distant metastasis. Invasion was defined as islands of less well-differentiated epithelial cells within the smooth muscle stroma that were not associated with the epithelium lining the gland. The authors emphasized the need to differentiate invasive epithelial cells from herniation of dysplastic epithelial cells associated with the grade 4 and 5 adenomas (Suttie et al. 2003).
In 2004, the Bar Harbor Meeting of the Mouse Models of Human Cancer Consortium Prostate Pathology Committee released a consensus report that reviewed and classified the pathologic changes in different transgenic mouse models of prostate cancer, including TRAMP mice (Shappell et al. 2004). The pathologic changes that were described in the prostates of C57BL/6 TRAMP and C57BL/6 TRAMP × FvB mice included epithelial hyperplasia, combined epithelial and stromal hyperplasia, mouse PIN (mPIN), microinvasive carcinoma, and invasive carcinoma. Invasive carcinoma included well-, moderately, and poorly differentiated adenocarcinomas, as well as neuroendocrine carcinomas. It was noted that the combined epithelial and stromal hyperplasia described in this report may resemble phyllodes tumor of the human breast when the stroma was exuberant and edematous. Hyperplasia was classified as either focal or diffuse based on whether 50% or more of the glands were involved. In both epithelial hyperplasia and mPIN, the cells may exhibit nuclear atypia (such as hyperchromasia, anisokaryosis, and increased mitotic index) and often stratify and form tufts, papillary or polypoid projections, and/or cribriform patterns. mPIN was differentiated from hyperplasia based on the opinion that mPIN begins focally and progresses with regard to the proportion of each gland involved, number of glands affected, and the severity of the observed atypia, whereas hyperplasia is nonprogressive and may involve the whole gland simultaneously. Moreover, mPIN was not classified as either low or high grade because the histologic features of mPIN that are associated with progression to invasion had not been defined. Microinvasive carcinoma was indicated by the penetration of the basement membrane by carcinoma cells in a gland with mPIN. Invasive carcinoma was characterized by desmoplasia associated with the epithelial cells that invaded into the smooth muscle surrounding the glands, adequate distance separating the epithelial cells within the smooth muscle from those lining the gland, or evidence that the basement membrane was breached using immunohistochemistry, special stains, or electron microscopy. Invasive was distinguished from microinvasive carcinoma based on the degree of invasion into the surrounding stroma or tissues, as well as evidence of metastasis. Moreover, the importance of differentiating invasive epithelial cells from herniated epithelial cells was emphasized. Invasive carcinomas were categorized as well-, moderately, and poorly differentiated adenocarcinomas and neuroendocrine carcinomas. The degree of differentiation was distinguished by the extent of gland formation by the neoplastic cells, with lesser differentiation associated with the replacement of neoplastic glands by solid sheets of cells. Neuroendocrine carcinomas were defined as invasive carcinomas that exhibited histologic, immunohistochemical, and ultrastructural features of neuroendocrine differentiation. Specifically, these lesions would be composed of solid sheets of cells with rosettes or cribriform patterns and the absence of glands. The cells should stain for at least one immunohistochemical marker of neuroendocrine differentiation, such as synaptophysin or chromogranin A. Dense, neuroendocrine type, secretory granules should be visible by electron microscopy (Shappell et al. 2004).
The lesions described in the grading scheme presented by Chiaverotti et al. in 2008 included mild, moderate, and severe atypical hyperplasia of Tag (with Tag denoting that the mice express the SV40 T antigen-containing transgene), papillary adenoma, adenocarcinoma (well, moderately, and poorly differentiated), and neuroendocrine carcinoma in C57BL/6 TRAMP and C57BL/6 TRAMP × FvB mice. Atypical hyperplasia of Tag was the term used instead of PIN because these authors concluded that the proliferative lesions in the TRAMP mice did not conform to their definition of PIN. These authors differentiate PIN, a lesion in which only the epithelium is affected, from atypical hyperplasia of Tag, in which both the epithelium and stroma are involved. Low-grade PIN was described as epithelium forming plaque-like lesions one- to two-cells thick and high-grade PIN as epithelium forming cribriform structures, as well as plaques that may be multiple-cell-layers thick. In addition, it was the opinion of the authors that the proliferative lesions in TRAMP mice did not progress to invasion, so the lesions (which others have defined as PIN) were named atypical hyperplasia of Tag. Mild atypical hyperplasia of Tag was described as epithelial crowding with cells having increased nuclear to cytoplasmic ratios and loss of cell polarity. The focal areas of cell crowding progressed to affect the whole gland diffusely with concurrent smooth muscle hyperplasia. In moderate atypical hyperplasia of Tag, the epithelial proliferation and dysplasia were greater with herniation, but not invasion, of hyperplastic cells into the smooth muscle surrounding the glands. Herniation of dysplastic epithelial cells was distinguished from invasion by the absence of a stromal reaction (replacement of the stromal smooth muscle component by reactive fibroblasts and myofibroblasts). In severe atypical hyperplasia of Tag, the proliferative epithelium formed cribriform structures. Papillary adenomas were defined as benign proliferations of epithelium and associated stroma that protruded as polypoid masses into the lumen and were similar to phyllodes-like lesions described by others. Neuroendocrine carcinomas were composed of solid sheets of highly anaplastic cells that were synaptophysin positive and occasionally weakly positive for the androgen receptor. The authors defined well-differentiated adenocarcinomas as neoplasms with apparent epithelial origins and distinct glandular morphology and those less well differentiated as having a more solid morphology but, importantly, as being negative for synaptophysin. Moreover, invasion (as determined by a stromal reaction) and metastasis were considered features of adenocarcinomas. The authors reported that no adenocarcinomas occurred in the TRAMP mice, and they speculated that the observed neuroendocrine carcinomas arose from distinct neuroendocrine stem cells within the prostate and not from progression of atypical hyperplasia of Tag (Chiaverotti et al. 2008).
Rationale for a Refined Grading Scheme
It may be argued that the differences among grading schemes are not important for determining whether a drug succeeds in preventing neoplasia in TRAMP mice. However, we suggest that there are significant differences among the available schemes that necessitate the development of a concise and inclusive grading scheme. Moreover, we believe that refinements can be made in distinguishing lesions that can enhance assessments of chemopreventive interventions in this model. Specifically, our rationales for creating a refined grading scheme for prostatic lesions in TRAMP mice are threefold. First, we sought to establish criteria for distinguishing among low-, moderate-, and high-grade PIN. Such distinctions would make it possible to determine if a drug alters the severity of PIN, which may signify that it slows the progression of lesions in the TRAMP model, an important endpoint in a chemoprevention study. Second, we wanted to define invasion to permit differentiation of herniated dysplastic epithelial cells (high-grade PIN) from epithelial cells that have invaded into the underlying stroma (adenocarcinoma). Differentiating high-grade PIN, a preneoplastic lesion, from well-differentiated adenocarcinoma, a neoplastic lesion, is critical for determining if an intervention has successfully prevented progression to neoplasia in the TRAMP model. Criteria previously described for differentiating invasive cells from herniated cells include the presence of epithelial cells within the smooth muscle surrounding the gland (Kaplan-Lefko et al. 2003), a stromal reaction (Chiaverotti et al. 2008; Shappell et al. 2004), and the distance between cells outside of the gland and those within the acinus (Suttie et al. 2003; Shappell et al. 2004). We propose that classifying invasion based on the distance between the free epithelial cells within the smooth muscle surrounding the acinus and the epithelial cells lining the acinus is inconsistent since this distance will vary from section to section of gland examined. Third, we sought to incorporate a numerical grading system that allows simultaneous assessment of lesion severity and extent of the lobe involvement. An ideal grading scheme should account for not only lesion type but also its distribution, since the extent of lobe involvement is a measure of lesion progression. A lesion that involves more of a gland is assumed to have progressed further and therefore is interpreted as more severe. Combining the type of lesion with an indication of its distribution to generate a numerical value will facilitate the assessment of lesion severity and permit statistical evaluation. Such an approach has been proposed by Suttie et al. (2003).
The Refined Grading Scheme
Each of the four lobes (dorsal, ventral, lateral, anterior) of the prostates of C57BL/6 TRAMP × FvB mice is assessed individually and assigned two grades each, ranging from 0 to 7. The first grade represents the most severe lesion within that lobe, with normal prostate as the least severe (grade 0) and poorly differentiated carcinoma as the most severe lesion (grade 7). The second grade, using the same scale, identifies the most common lesion in the lobe, which is defined as the lesion with an extent of lobe involvement that is greater than or equal to that of the most severe lesion. By assessing both the most severe lesion and most common lesion, we believe that a more complete picture of disease status can be obtained. In addition, by accounting for both of these lesions, the refined grading scheme begins to approximate the Gleason system used to grade prostate cancer in men (Kumar et al. 2005).
The lesion grades for the refined scheme are as follows. Grade 0 is normal prostate. Grades 1, 2, and 3 represent low-, moderate-, and high-grade PIN, respectively. Although different terms have been used to describe the preneoplastic lesions in the prostates of TRAMP mice, such as hyperplasia (Suttie et al. 2003), atypical hyperplasia of Tag mice (Chiaverotti et al. 2008), and PIN (Kaplan-Lefko et al. 2003), we prefer the term PIN because it implies progression to adenocarcinoma. Grade 4 includes phyllodes-like lesions. Grades 5 and 6 represent well-and moderately differentiated adenocarcinomas, respectively. Grade 7 is poorly differentiated carcinoma, which may have neuroendocrine features.
After the most severe and most common lesions within a lobe are identified, the distributions of each of these lesions within the individual lobe are determined and described as focal, multifocal, or diffuse. Focal indicates there are fewer than three foci within the lobe. Lesions are described as multi-focal if there are three or more foci within the lobe, with less than 50% of the lobe containing the lesion of interest. Finally, lesions are diffuse if greater than 50% of the lobe is affected.
Adjusting the lesion grades to include an indication of distribution provides two adjusted scores, one for the most severe lesion and the other for the most common, ranging from 0 (normal) to 21 (diffuse, poorly differentiated carcinoma; Table 1). These adjusted scores are then added to obtain a sum that reflects the most severe lesion and its distribution and the most common lesion and its distribution (sum of the adjusted lesion scores). If the most severe lesion is also the most common lesion, then the adjusted score for the most severe lesion is simply doubled to obtain the sum of the adjusted lesion scores. If most of the prostate is normal, then the sum is simply the adjusted lesion score for the most severe lesion plus zero. This refined grading scheme incorporates both the most severe and most common lesions, both of which contribute to the pathology present within a lobe. A detailed description of the seven grades follows.
Table 1.
Determination of the adjusted lesion score from the lesion grade and distribution.
| Lesion grade | Distribution | Adjusted lesion score |
|---|---|---|
| 0 | Diffuse | 0 |
| 1 | Focal | 1 |
| 1 | Multifocal | 2 |
| 1 | Diffuse | 3 |
| 2 | Focal | 4 |
| 2 | Multifocal | 5 |
| 2 | Diffuse | 6 |
| 3 | Focal | 7 |
| 3 | Multifocal | 8 |
| 3 | Diffuse | 9 |
| 4 | Focal | 10 |
| 4 | Multifocal | 11 |
| 4 | Diffuse | 12 |
| 5 | Focal | 13 |
| 5 | Multifocal | 14 |
| 5 | Diffuse | 15 |
| 6 | Focal | 16 |
| 6 | Multifocal | 17 |
| 6 | Diffuse | 18 |
| 7 | Focal | 19 |
| 7 | Multifocal | 20 |
| 7 | Diffuse | 21 |
Grade 0: Normal: Prostate glands are lined by a monolayer of cuboidal to columnar epithelial cells with basally oriented nuclei. There may be mild to moderate in-folding of epithelial cells in the dorsal and anterior prostate lobes and rare in-folding in the ventral and lateral prostate lobes.
Grade 1: Low-grade PIN: There is crowding and occasional stratification of prostate epithelial cells with an increased nuclear to cytoplasmic ratio (Figure 1A). There may be rare short papillary projections of hyperplastic epithelium into the glandular lumen in the lateral and ventral lobes (Figure 1B). Cytoplasmic and nuclear atypia are minimal.
Grade 2: Moderate-grade PIN: Similar to low-grade PIN but there is more frequent prostate epithelial cell stratification. Hyperplastic cells may be arranged as short to tall papillary projections protruding into glandular lumen (Figure 2A, B); rarely, the hyperplastic epithelial cells form a cribriform pattern. There may be mild hyperplasia of smooth muscle surrounding the glands. Occasional herniation of hyperplastic epithelial cells into the underlying smooth muscle may be observed.
Grade 3: High-grade PIN: Like low- and moderate-grade PIN, there is cell crowding, cell stratification, and an increased nuclear-to-cytoplasmic ratio. Hyperplastic cells project into the lumen as papillary projections or form a cribriform pattern (Figure 3A). Mitotic figures may be frequent. There is frequent herniation of epithelial cells into hyperplastic smooth muscle surrounding the glands (Figure 3B, C). Herniated dysplastic glands have no stromal reaction. Proliferating epithelium may fill and/or expand the lumen of the gland. The hyperplastic epithelium filling the lumen may consist of small hyperplastic acini without associated stroma (Figure 3D). Alternatively, the epithelium expanding the lumen may form a discrete mass that is continuous with the proliferative epithelium lining the lumen of the gland (Figure 3E, F). The proliferating mass of epithelium may contain associated tightly packed stroma. In this refined grading scheme, such lesions are defined as high-grade PIN and not adenomas. There may be mild to moderate hyperplasia of the smooth muscle surrounding the individual glands with high-grade PIN. In the ventral and lateral lobes, there may be minimal to mild proliferations of fibroblasts intervening between and surrounding the glands (Figure 3D). There is no evidence of invasion or metastasis.
Grade 4: Phyllodes-like tumor: These lesions are most commonly observed in the dorsal or anterior lobes. They consist of papillary projections of loose stroma with loosely arranged stellate mesenchymal cells (Figure 4A). The epithelium overlying the stroma of the phyllodes-like tumor is cuboidal to columnar, with minimal to no nuclear or cytoplasmic atypia and rare mitotic figures (Figure 4B). The main component of a phyllodes-like tumor is stroma. Previous descriptions of these tumors by others suggest that these tumors are not malignant (Tani et al. 2005) and likely represent a variant of an adenoma.
Grade 5: Well-differentiated adenocarcinoma: The lumen of the affected gland contains a mass of epithelial cells forming well-differentiated acini or tubule-like structures. The epithelial cells frequently have nuclear and cytoplasmic atypia and a high mitotic index. The acini may be separated by a mild amount of tightly packed stroma. Well-differentiated adenocarcinomas are differentiated from high-grade PIN by the presence of invasion, which is evident as invasion of epithelial cells into the underlying smooth muscle, with cells crossing the basement membrane and replacement of the normal smooth muscle surrounding the gland by reactive fibroblasts and myoepithelial cells in the area of invasion (Figures 5A–C). This stromal reaction may be exuberant and encompass adjacent glands or may be more focused around the lone invading epithelial cells. It must be noted that there may be a minimal to mild amount of fibrous connective tissue with fibroblasts and myoepithelial cells in the interglandular regions of the ventral and lateral lobes with high-grade PIN. However, if cells do not penetrate through the basement membrane in these areas, this is considered high-grade PIN and not invasion.
Grade 6: Moderately differentiated adenocarcinoma: The lumen of the affected gland contains a mass of epithelial cells, some of which form acini or tubules (Figure 6A). The epithelial cells frequently have nuclear and cytoplasmic atypia and a high mitotic index. The epithelial cells may be separated by a mild amount of tightly packed stroma. There is invasion of epithelial cells into the underlying smooth muscle, with cells crossing the basement membrane and the replacement of the normal smooth muscle by reactive fibroblasts.
Grade 7: Poorly differentiated carcinoma (neuroendocrine--type): The tumors consist of solid sheets of polygonal to elongated cells with high mitotic indices and frequent nuclear and cytoplasmic atypia (Figure 6B). Although the neoplastic cells do not form tubules or acini, they may entrap normal-appearing glands.
Figure 1.
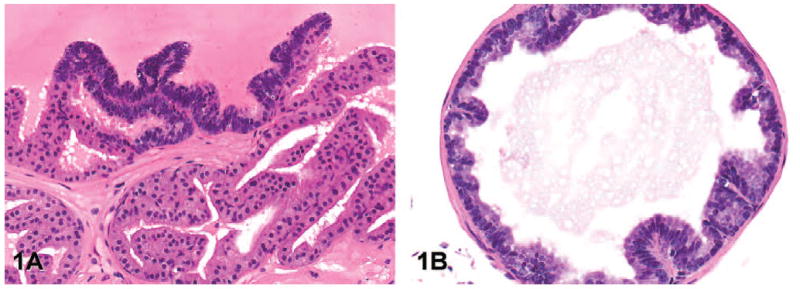
Representative images of low-grade prostatic intraepithelial neoplasia (lesion grade 1) in transgenic adenocarcinoma of the mouse prostate. (A) Note the focal cell stratification, increased nuclear to cytoplasmic ratio, and crowding of the epithelial cells (anterior lobe, hematoxylin and eosin [H&E], magnification 200×). (B) Few, short papillary proliferations of hyperplastic epithelium project into the lumen (ventral lobe, H&E, 200×).
Figure 2.
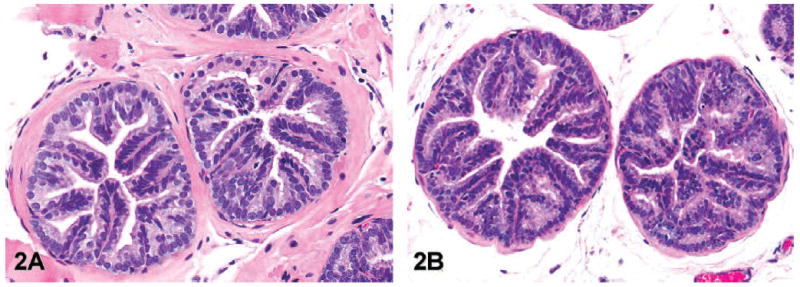
Representative images of moderate-grade prostatic intraepithelial neoplasia (lesion grade 2) in transgenic adenocarcinoma of the mouse prostate. Note the more prominent papillary projections of hyperplastic epithelial cells that project into the lumen in the dorsal (A, hematoxylin and eosin [H&E], 200×) and ventral lobes (B, H&E, magnification 100×).
Figure 3.
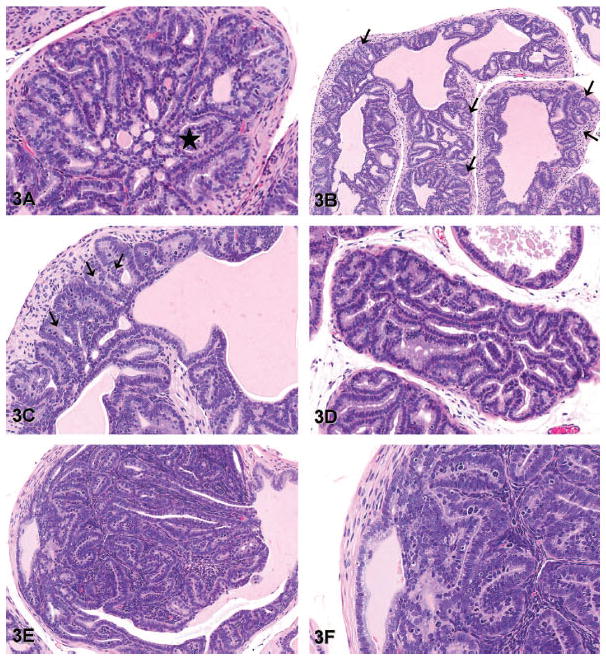
Representative images of high-grade prostatic intraepithelial neoplasia (lesion grade 3) in transgenic adenocarcinoma of the mouse prostate. (A) The epithelium forms a cribriform pattern filling the lumen of the gland (star). Note that the epithelium adjacent to the area of the cribriform pattern, as well as the epithelium of adjacent glands, forms tall papillary projections extending into the lumens (dorsal lobe, hematoxylin and eosin [H&E], 100×). (B) There is frequent herniation of clusters of dysplastic epithelial cells (arrows) into the underlying smooth muscle capsule (dorsal lobe, H&E, 80×). (C) Higher magnification of the image in panel B clearly shows herniation of epithelial cells into underlying smooth muscle (arrows) without any evidence of stromal reaction (dorsal lobe, H&E, 200×). (D) Acini of hyperplastic epithelial cells fill the lumen of the gland, and minimal proliferation of fibroblasts between adjacent glands is evident (ventral lobe, H&E, 100×). (E) Note the mass of proliferating epithelial cells and tightly compacted stroma protruding into the lumen. There is no distinction between the proliferating epithelium and the adjacent epithelium lining the gland (dorsal lobe, H&E, 100×). (F) Higher magnification of the image in panel E shows the smooth muscle capsule surrounding the gland is intact and there is no evidence of invasion. The stroma between acini of the proliferating dysplastic epithelium is tightly compacted and a minor component of the lesion (H&E, 200×).
Figure 4.
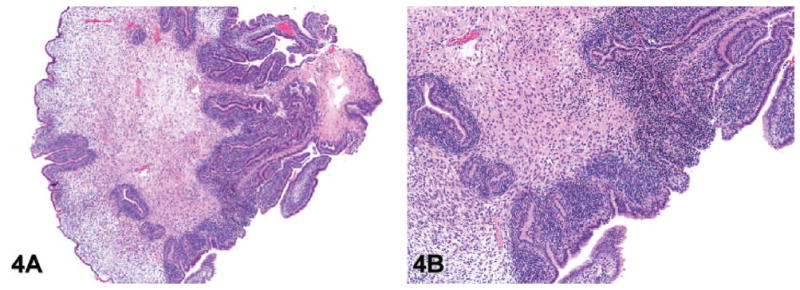
Representative images of phyllodes-like tumor (lesion grade 4) in transgenic adenocarcinoma of the mouse prostate. (A) Phyllodes-like tumor in the anterior lobe of the prostate (hematoxylin and eosin [H&E], 20×). (B) Higher magnification of the image in panel A shows that the phyllodes-like tumor consists of papillary projections of loose stroma with loosely arranged stellate mesenchymal cells and epithelial cells with minimal or no nuclear or cytoplasmic atypia and rare mitotic figures (H&E, 80×).
Figure 5.
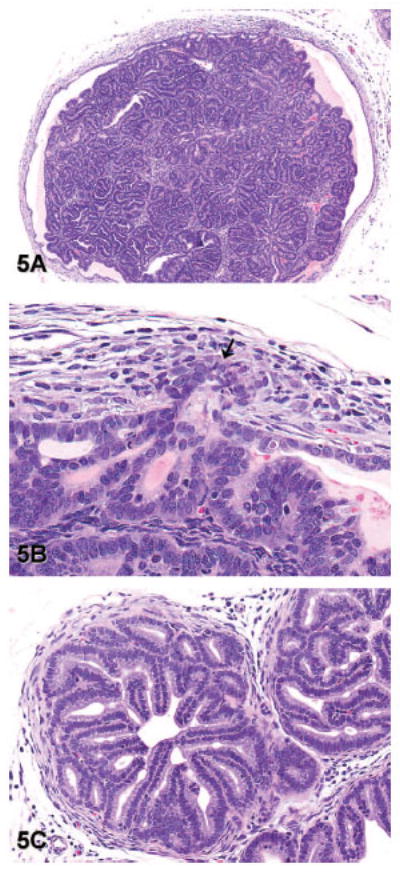
Representative images of well-differentiated adenocarcinoma (lesion grade 5) in transgenic adenocarcinoma of the mouse prostate. (A) Well-differentiated adenocarcinoma forming a mass of epithelial cells and acini that expands the lumen of a gland from the dorsal lobe of the prostate. Note that this gland is no longer surrounded by a complete or distinct smooth muscle capsule (hematoxylin and eosin [H&E], 40×). (B) Higher magnification of the image in panel A shows invasion of epithelial cells through the basement membrane and into the adjacent stroma (arrow). A stromal reaction in response to invasion is evident as reactive fibroblasts and myoepithelial cells surrounding the invading cells (H&E, 400×). (C) Note the exuberant stromal reaction and replacement of the smooth muscle capsule by reactive fibroblasts in this focus of well-differentiated adenocarcinoma from the lateral prostate (H&E, 200×).
Figure 6.
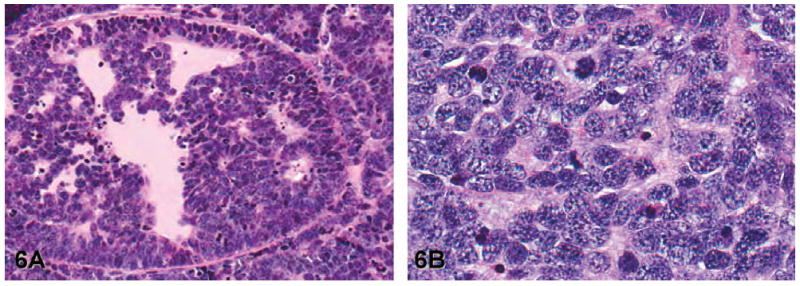
Representative images of moderately differentiated adenocarcinoma (lesion grade 6) and poorly differentiated carcinoma (lesion grade 7) in the lateral lobe of transgenic adenocarcinoma of the mouse prostate. (A) Moderately differentiated adenocarcinoma: Note the small acinar-like structures formed by cells exhibiting varying degrees of nuclear and cytoplasmic atypia (hematoxylin and eosin [H&E], 100×). (B) Poorly differentiated carcinoma: Note the solid sheets of polygonal to elongated cells, a high mitotic index, frequent nuclear and cytoplasmic atypia, and the lack of glands (H&E, 400×).
Utilization of the Grading Scheme
To demonstrate the utility of the refined grading scheme, we report the average sum of adjusted lesion scores for the lobes of the prostates of 42 intact C57BL/6 TRAMP × FvB mice between the ages of 18 and 24 wk. Previous use of TRAMP mice in our laboratory have shown the advantage of using a 24-wk endpoint for a chemoprevention study (Sargeant et al. 2008). Before the age of 18 wk, few TRAMP mice will have developed carcinomas (Gingrich et al. 1999, 1996); thus, a study having an endpoint prior to this age will be limited in its ability to determine whether an agent can prevent prostate tumorigenesis. In fact, our laboratory has shown that only 40% of intact C57BL/6 TRAMP × FvB mice develop malignant tumors by 18 wk of age (Sargeant et al. 2007). Conducting a chemopreventive study past 24 wk of age is not recommended, since after that age, most control mice will have developed poorly differentiated prostate carcinomas (Gingrich et al. 1996, 1999), necessitating humane euthanasia and thereby limiting the number of mice available for study.
Materials and Methods
TRAMP mice (C57BL/6 TRAMP × FvB) were bred and housed as previously reported (Sargeant et al. 2007), and the presence of the transgene was confirmed by polymerase chain reaction. Mice received a standard rodent diet and water ad libitum. Mice were euthanized between 18 and 24 wk of age by carbon dioxide asphyxiation. All procedures were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee of The Ohio State University. At necropsy, the four lobes of each prostate were collected, microdissected, isolated, and placed in 10% formalin and processed as previously described (Sargeant et al. 2007). Hematoxylin and eosin–stained sections of prostates were examined using light microscopy. Lesions were described, and the sum of the adjusted lesion scores of each lobe was calculated for each mouse using the grading scheme described above. The lesion grades are presented as the mean (± standard deviation [SD]) of the sums of the adjusted lesion scores of each lobe for all 42 mice.
Results
The individual lobes of the prostates from 42 intact male TRAMP mice were examined histologically, the most severe and most common lesions in each lobe were determined and graded, and adjusted lesion scores were calculated. The results are summarized in Tables 2 and 3, which show the lesion types identified as most severe and most common and their incidences in each lobe of the prostate. As shown in Table 2, poorly differentiated carcinoma was the most severe lesion observed in all four prostatic lobes. In the ventral, lateral, and dorsal lobes, poorly differentiated carcinoma was the most severe lesion in the majority of mice, with incidences of 66.7%, 64.3%, and 52.4%, respectively. In contrast, in the anterior lobe, high-grade PIN was the lesion most frequently observed to be the most severe (52.4% of mice), with poorly differentiated carcinoma being the second most frequent lesion (38.1%). A similar pattern was observed for the most common lesions (Table 3). In the ventral, lateral, and dorsal lobes, poorly differentiated carcinoma was the most common lesion, with incidences of 64.3%, 64.3%, and 52.4%, respectively. In the anterior prostate, however, normal prostatic morphology was observed to be the most common histologic feature in the majority of mice (59.5%).
Table 2.
Incidences (%)a of the prostate lesions identified as most severe in each lobe of the prostates of TRAMP mice.
| Lesion | Prostate lobe
|
|||
|---|---|---|---|---|
| Ventral | Lateral | Dorsal | Anterior | |
| Normal | 0b | 0b | 0b | 2.4 |
| Low-grade PIN | 4.8 | 0b | 0b | 4.8 |
| Moderate-grade PIN | 14.3 | 7.1 | 0b | 0b |
| High-grade PIN | 2.4 | 2.4 | 40.5 | 52.4 |
| Phyllodes-like | 0c | 0c | 2.4 | 2.4 |
| Well-differentiated adenocarcinoma | 9.5 | 23.8 | 4.8 | 0c |
| Moderately differentiated adenocarcinoma | 2.4 | 2.4 | 0c | 0c |
| Poorly differentiated carcinoma | 66.7 | 64.3 | 52.4 | 38.1 |
PIN = prostatic intraepithelial neoplasia; TRAMP = transgenic adenocarcinoma of the mouse prostate.
Incidence expressed as a percentage of a total of 42 mice.
Lesions of this type were observed but did not represent the most severe lesion in this lobe in any of the mice.
No lesions of this type were observed in this lobe.
Table 3.
Incidences (%)a of the prostate lesions identified as most common in each lobe of the prostates of TRAMP mice.
| Lesion | Prostate lobe
|
|||
|---|---|---|---|---|
| Ventral | Lateral | Dorsal | Anterior | |
| Normal | 4.8 | 0b | 0b | 59.5 |
| Low-grade PIN | 9.5 | 0b | 0b | 0b |
| Moderate-grade PIN | 14.3 | 14.3 | 2.4 | 0b |
| High-grade PIN | 2.4 | 19.0 | 45.2 | 2.4 |
| Phyllodes-like | 0c | 0c | 0b | 0b |
| Well-differentiated adenocarcinoma | 2.4 | 2.4 | 0b | 0c |
| Moderately differentiated adenocarcinoma | 2.4 | 0b | 0c | 0c |
| Poorly differentiated carcinoma | 64.3 | 64.3 | 52.4 | 38.1 |
PIN = prostatic intraepithelial neoplasia; TRAMP = transgenic adenocarcinoma of the mouse prostate.
Incidence expressed as a percentage of a total of 42 mice.
Lesions of this type were observed but did not represent the most common lesion in this lobe in any of the mice.
No lesions of this type were observed in this lobe.
This pattern of lesion severity and distribution among the prostatic lobes of TRAMP mice was reflected in the average sums of the adjusted lesion scores. For the ventral, lateral, and dorsal lobes, the average sums of the adjusted lesion scores were 32.1 ± 14.7, 34.0 ± 11.2, and 30.9 ± 12.1 (mean ± SD), respectively, while that of the anterior lobe was 20.6 ± 17.2.
Discussion
Based on our examination of the prostate glands of 42 intact TRAMP mice between the ages of 18 and 24 wk, poorly differentiated carcinoma was the most severe lesion observed in all four prostatic lobes. However, only in the ventral, lateral, and dorsal lobes was poorly differentiated carcinoma the lesion most frequently identified as most severe and most common (Tables 2 and 3). In the anterior lobe, although poorly differentiated carcinoma was also present, high-grade PIN was the lesion observed to be the most severe lesion in the largest proportion of mice (52.4%). In addition, in the anterior lobe, the most common “lesion” in the majority of mice was no lesion at all (i.e., normal prostate histology). These results are consistent with the previously reported descriptions of tumorigenic progression in TRAMP mice (Kaplan-Lefko et al. 2003; Gingrich et al. 1996, 1999; Hurwitz et al. 2001).
Among the other published grading schemes that we reviewed (Table 4), that proposed by Suttie et al. (2003) is the most similar to the refined scheme described here. The Suttie scheme recommends evaluating each lobe of the prostate and assigning, based on the most severe lesion within the lobe, a grade from 1 to 6. The grades 1 through 3 represent varying degrees of hyperplasia, grades 4 and 5 represent adenomas, and grade 6 represents adenocarcinomas. The distribution of the lesion within the lobe is then estimated as focal, multifocal, or diffuse and is combined with the lesion grade to give a distribution-adjusted lesion grade, which ranges from 0 to 18. The versatility of a grading scheme that combines lesion severity and distribution into a single score is commendable. However, to characterize the lesions within a lobe and obtain a score that is indicative of the most significant lesions (i.e., not only the most severe but also the most common), we assigned numerical values for both the severity and distribution of both the most severe and most common lesions to generate a score referred to above as the sum of the adjusted lesion scores. Another important distinction between the Suttie scheme and our refined scheme is how invasion is defined. Suttie et al. define invasion as islands of epithelial cells within the smooth muscle stroma that are less well differentiated and not closely associated with the epithelium lining the gland. We prefer a definition of invasion that does not rely on an estimation of the distance between epithelial cells in the smooth muscle and the glandular epithelium, since this distance may vary between tissue sections with plane of cut. Thus, we identify invasion based on the presence of stromal reaction associated with epithelial cells present within the smooth muscle capsule. Also, the refined grading scheme assigns only one grade for adenomas, instead of two. The histologic descriptions of adenomas in the Suttie scheme are similar to our definition of high-grade PIN. Finally, the refined scheme segregates carcinomas into three distinct groups (well, moderately, and poorly differentiated) since a treatment that shifts prostate phenotype from poorly differentiated carcinoma to well-differentiated adenocarcinoma could be clinically important.
Table 4.
Summary of published and proposed refined grading schemes for the prostate glands of TRAMP mice.
| Grading Scheme | Number of Different Lesions | Number of Preneoplastic Lesions | Terminology for Preneoplastic Lesions | Definition of Invasion | Numerical Score |
|---|---|---|---|---|---|
| Gingrich et al. (1999) | 5 | 2 | PIN | Epithelial cells within the stroma-surrounding glands; rounded nuclei present within the fibromuscular stroma surrounding glands | No |
| Kaplan-Lefko et al. (2003) | 5 | 1 | PIN | Epithelial cells within smooth muscle stroma; increased number of small glandular structures with associated desmoplasia | No |
| Suttie et al. (2003) | 6 | 3 | Hyperplasia | Islands of epithelial cells within the smooth muscle stroma appear less well differentiated and are not closely associated with the outline of epithelium lining the gland | Yes: distribution-adjusted| lesion grade |
| Bar Harbor Meeting, 2004 | 5 | 1 | Mouse PIN (mPIN) | Penetration of the basement membrane by epithelial cells; recognized based on presence of desmoplasia adjacent to the epithelial cells that have invaded into the smooth muscle, adequate distance separating the cells within the smooth muscle from the epithelial cells lining the gland, or clear evidence of the basement membrane being penetrated (determined using immunohistochemistry, special stains, or electron microscopy) | No |
| Chiaverotti et al. (2008) | 6 | 3 | Atypical hyperplasia of Tag | Presence of stromal reaction to the invading epithelial cells | No |
| Berman-Booty et al. (2011) | 7 | 3 | PIN | Presence of epithelial cells in the underlying smooth muscle, with cells crossing the basement membrane and replacement of the normal smooth muscle surrounding gland by reactive fibroblasts and myoepithelial cells | Yes: sum of the adjusted lesion scores |
The grading scheme described by Gingrich et al. (1999) defined low-grade PIN, high-grade PIN, well-differentiated adenocarcinoma, moderately differentiated adenocarcinoma, and poorly differentiated adenocarcinoma as the lesions present in the prostates of TRAMP mice. The differences between the two grades of PIN described in the Gingrich scheme and the three grades of PIN in the refined grading scheme are subtle. However, we propose that there is a distinct intermediate grade of PIN between low- and high-grade PIN. This could be helpful in defining the effects of chemopreventive agents that may target the PIN stage of prostate cancer progression. The Gingrich scheme defined invasion as epithelial cells invading into the stroma surrounding glands or as rounded nuclei within hypertrophied and hyperplastic fibromuscular stroma surrounding glands. The rounded nuclei were reported to be immunohistochemically positive for the T antigen, suggesting that they belonged to transformed epithelial cells (Gingrich et al. 1999). The presence of nuclei that are immunopositive for the T antigen is a novel and interesting criterion for invasion. However, without demonstration of T antigen immunoreactivity, we assume these nuclei represent hyperplastic smooth muscle cells that frequently surround glands with moderate- and high-grade PIN lesions. For simplicity and consistency, we propose a scheme that does not rely on immunohistochemistry to identify invasion.
The grading scheme proposed by Kaplan-Lefko et al. (2003) classified the lesions in the prostate glands of TRAMP mice as either PIN, well-differentiated carcinoma, moderately differentiated carcinoma, poorly differentiated carcinoma, or phylloides-like lesions. Although this scheme provides a simple and straightforward way to describe the lesions, in our view, the variability in the cytological and architectural changes that occur in PIN necessitated the division of PIN into three categories to emphasize the progression of hyperplasia and dysplasia that occurs in the PIN lesions. In addition, the Kaplan-Lefko scheme does not address the issue of differentiating herniation of dysplastic cells from invasion. Photomicrographs accompanying the description of this scheme imply that herniation is equivalent to invasion.
In the descriptions of the prostatic lesions of TRAMP mice in the Consensus Report from the Bar Harbor Meeting of the Mouse Models of Human Cancer Consortium Prostate Pathology Committee (Shappell et al. 2004), the authors list hyperplasia (both epithelial alone and combined epithelial and stromal), adenomas and papillomas, mouse PIN (mPIN), microinvasive carcinoma, and invasive carcinoma (which includes well differentiated, moderately differentiated, and poorly differentiated neuroendocrine carcinomas). Among the grading schemes reviewed, this is the only one that differentiated hyperplasia from mPIN. Since PIN progresses to adenocarcinoma in TRAMP mice, we felt it unnecessary to describe a separate lesion of hyperplasia. While our definition of PIN is consistent with the authors’ definition of mPIN, the refined scheme identifies different categories of PIN. The Consensus Report defined invasion based on the presence of desmoplasia associated with epithelial cells that have invaded into the smooth muscle surrounding the glands, “adequate” distance separating the invasive epithelial cells from the gland, or unequivocal evidence that the basement membrane was penetrated by epithelial cells confirmed by immunohistochemistry, special stains, or electron microscopy. We agree that penetration of the basement membrane and a stromal reaction associated with the epithelial cells within the smooth muscle support a diagnosis of invasion as we have delineated in our scheme, but we prefer to not use the distance between epithelial cells within the smooth muscle stroma and the gland as a criterion for invasion, since this may vary between sections.
The grading scheme used by Chiaverotti et al. (2008) described mild, moderate, and severe atypical hyperplasia of Tag, papillary adenoma, adenocarcinoma (well, moderately, and poorly differentiated) and neuroendocrine carcinoma as the lesions observed in TRAMP mice. Although the terminology differs, the description of atypical hyperplasia of Tag mice is similar to our description of PIN. Chiaverotti et al. suggest that the term PIN should not be used to describe the lesions in TRAMP mice, since PIN implies epithelial hyperplasia and dysplasia with no stromal involvement. We prefer to use the term PIN because we and others believe that it is descriptive of the natural progression of these lesions. Nonetheless, we acknowledge that the PIN we describe in the prostates of TRAMP mice is different from that observed in human prostates. As for defining invasion, the Chiaverotti scheme, like our refined scheme, differentiates invasion from herniation of dysplastic epithelial cells based on the presence of a stromal reaction.
We have compared our new, refined grading scheme with selected examples of other reported schemes in TRAMP mice. The refined grading scheme was designed to be especially useful for chemoprevention studies. The proposed advantages of the refined grading scheme include distinctions among low-, moderate-, and high-grade PIN, which will enable studies to determine if a treatment alters the severity of PIN and slows the progression of preneoplastic lesions in the TRAMP model. In addition, the refined grading scheme provides a useful definition of invasion and permits differentiation of herniated dysplastic epithelial cells from epithelial cells that have invaded into the underlying stroma. Differentiating high-grade PIN from well-differentiated carcinoma with invasion is imperative for determining if a treatment prevents progression to neoplasia in the TRAMP model. Finally, the refined grading scheme assigns numerical values to lesion types and their distributions and combines the adjusted scores that represent the most severe lesion and the most common lesion and their respective distributions. By taking into consideration the most severe and most common lesions and their distributions, the refined scheme allows for the progression of lesions within a lobe to be fully assessed.
Although controversy still exists regarding the assessment, classification, and progression of the lesions that occur in the prostates of TRAMP mice, we believe that the discussions and debates that arise from it are essential for better understanding and use of this model. We anticipate that the readers will find our review of available grading schemes informative and that further discussion will be stimulated. We believe that our refined grading scheme is a valuable addition to the grading schemes currently available to investigators. We hope that other investigators will accept our scheme’s utility for fully evaluating the prostate lesions of TRAMP mice.
Acknowledgments
This work was supported by a T-32 Institutional National Research Service Award in Mouse Models of Human Disease (Ruth L. Kirschstein-NRSA; T32RR007073) to L.D.B.-B. and NIH grants (CA135560, CA112250, and PC074151) to C.-S.C.
Abbreviations
- PIN
prostatic intraepithelial neoplasia
- TRAMP
transgenic adenocarcinoma of the mouse prostate
Footnotes
The authors declared no potential conflicts of interests with respect to the authorship and/or publication of this article.
References
- Bonkhoff H. Neuroendocrine differentiation in human prostate cancer: morphogenesis, proliferation and androgen receptor status. Ann Oncol. 2001;12:S141–4. doi: 10.1093/annonc/12.suppl_2.s141. [DOI] [PubMed] [Google Scholar]
- Chiaverotti T, Couto SS, Donjacour A, Mao JH, Nagase H, Cardiff RD, Cunha GR, Balmain A. Dissociation of epithelial and neuroendocrine carcinoma lineages in the transgenic adenocarcinoma of mouse prostate model of prostate cancer. Am J Pathol. 2008;172:236–46. doi: 10.2353/ajpath.2008.070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingrich JR, Barrios RJ, Foster BA, Greenberg NM. Pathologic progression of autochthonous prostate cancer in the TRAMP model. Prostate Cancer Prostatic Dis. 1999;2:70–5. doi: 10.1038/sj.pcan.4500296. [DOI] [PubMed] [Google Scholar]
- Gingrich JR, Barrios RJ, Kattan MW, Nahm HS, Finegold MJ, Greenberg NM. Androgen-independent prostate cancer progression in the TRAMP model. Cancer Res. 1997;57:4687–91. [PubMed] [Google Scholar]
- Gingrich JR, Barrios RJ, Morton RA, Boyce BF, DeMayo FJ, Finegold MJ, Angelopoulou R, Rosen JM, Greenberg N. Metastatic prostate cancer in a transgenic mouse. Cancer Res. 1996;56:4096–102. [PubMed] [Google Scholar]
- Greenberg NM. Transgenic models for prostate cancer research. Urol Oncol. 1996;2:119–22. doi: 10.1016/s1078-1439(97)82844-x. [DOI] [PubMed] [Google Scholar]
- Greenberg NM, DeMayo F, Finegold MJ, Medina D, Tilley WD, Aspinall JO, Cunha GR, Donjacour AA, Matusik RJ, Rosen JM. Prostate cancer in a transgenic mouse. Proc Natl Acad Sci U S A. 1995;92:3439–43. doi: 10.1073/pnas.92.8.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg NM, DeMayo FJ, Sheppard PC, Barrios R, Lebovitz R, Finegold M, Angelopoulou R, Dodd JG, Duckworth ML, Rosen JM, Matusik RJ. The rat probasin gene promoter directs hormonally- and developmentally-regulated expression of a heterologous gene specifically to the prostate in transgenic mice. Mol Endocrinol. 1994;8:230–9. doi: 10.1210/mend.8.2.8170479. [DOI] [PubMed] [Google Scholar]
- Hurwitz AA, Foster BA, Allison JP, Greenberg NM, Kwon ED. The TRAMP mouse as a model for prostate cancer. Curr Protoc Immunol Chpt. 2001;20(Unit 20.5):20.25.21–3. doi: 10.1002/0471142735.im2005s45. [DOI] [PubMed] [Google Scholar]
- Kaplan-Lefko PJ, Chen TM, Ittman MM, Barrios RJ, Ayala GE, Huss WJ, Maddison LA, Foster BA, Greenberg NM. Pathobiology of autochthonous prostate cancer in a pre-clinical transgenic mouse model. Prostate. 2003;55:219–37. doi: 10.1002/pros.10215. [DOI] [PubMed] [Google Scholar]
- Kumar V, Abbas AK, Fausto N, editors. Robbins and Cotran: Pathologic Basis of Disease. Elsevier; Philadelphia, PA: 2005. [Google Scholar]
- Sargeant AM, Klein RD, Rengel RC, Clinton SK, Kulp SK, Kashida Y, Yamaguchi M, Wang X, Chen CS. Chemopreventive and bioenergetic signaling effects of PDK1/Akt pathway inhibition in a transgenic mouse model of prostate cancer. Toxicol Pathol. 2007;35:549–61. doi: 10.1080/01926230701338966. [DOI] [PubMed] [Google Scholar]
- Sargeant AM, Rengel RC, Kulp SK, Klein RD, Clinton SK, Wang YC, Chen CS. OSU-HDAC42, a histone deacetylase inhibitor, blocks prostate tumor progression in the transgenic adenocarcinoma of the mouse prostate model. Cancer Res. 2008;68:3999–4009. doi: 10.1158/0008-5472.CAN-08-0203. [DOI] [PubMed] [Google Scholar]
- Shappell SB, Thomas GV, Roberts RL, Herbert R, Ittman MM, Rubin MA, Humphrey PA, Sundberg JP, Rozengurt N, Barrios R, Ward JM, Cardiff RD. Prostate pathology of genetically engineered mice: definitions and classification. The Consensus Report from the Bar Harbor Meeting of the Mouse Models of Human Cancer Consortium Prostate Pathology Committee. Cancer Res. 2004;64:2270–305. doi: 10.1158/0008-5472.can-03-0946. [DOI] [PubMed] [Google Scholar]
- Suttie A, Nyska A, Haseman JK, Moser GJ, Hackett TR, Goldsworthy TL. A grading scheme for the assessment of proliferative lesions of the mouse prostate in the TRAMP model. Toxicol Pathol. 2003;31:31–8. doi: 10.1080/01926230390173842. [DOI] [PubMed] [Google Scholar]
- Tani Y, Suttie A, Flake GP, Nyska A, Maronpot RR. Epithelial-stromal tumor of the seminal vesicles in the transgenic adenocarcinoma of mouse prostate model. Vet Pathol. 2005;42:306–14. doi: 10.1354/vp.42-3-306. [DOI] [PubMed] [Google Scholar]
- Wechter WJ, Leipold DD, Murray ED, Quiggle D, McCracken JD, Barrios RS, Greenberg NM. E-7869 (R-flurbiprofen) inhibits progression of prostate cancer in the TRAMP mouse. Cancer Res. 2000;60:2203–08. [PubMed] [Google Scholar]


