Figure 1. An overview of the aberrant cell death and processing of self antigens in the pathogenesis of systemic lupus erythematosus (SLE) and possible therapeutic implications.
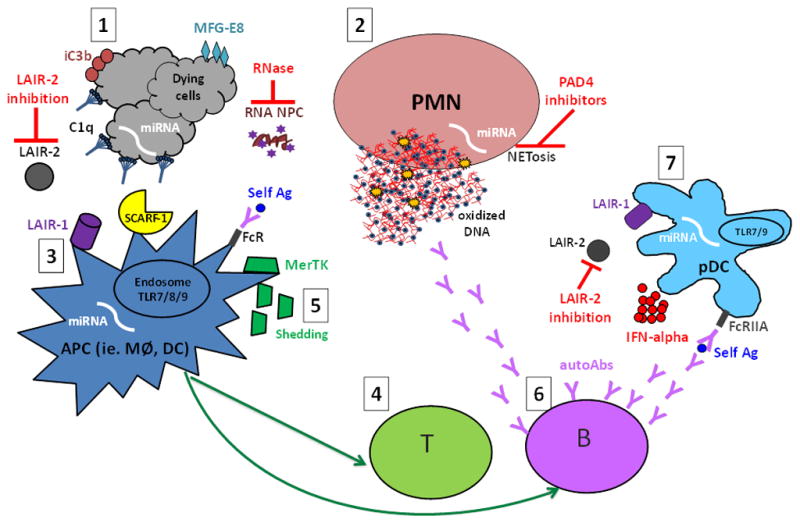
1. Aberrant cell death, i.e. increased or unwanted cell death, including NETosis and release of neutrophil (PMN) extracellular traps (NETs) (2), may provide increased availability and/or modified self antigens (self Ag), such as 8-OHG-carrying oxidized DNA- and RNA -containing nucleoprotein complexes (NPCs). Opsonins, in particular the complement components C1q and iC3b as well as MFG-E8, recognize dying cells or cell remnants and mark them for silent processing through interaction with, among others, the recently identified C1q receptors (LAIR-1 and SCARF-1 (3). In the absence of MFG-E8, self antigens are preferentially targeted for antigen presentation to T cells (4) instead of degradation. Further, MerTK-mediated swift uptake of early apoptotic cells (AC) was shown to be important to mediate the immunosuppressive effects of AC, while extracellular MerTK shedding seems to correlate with disease activity and has been proposed as a novel lupus biomarker (5). In genetically predisposed individuals these aberrations could lead to activation of autoreactive T and B cells and development of lupus autoimmunity, characterized by the production of autoantibodies (autoAbs) targeting NPCs (6). NPC-containing immune complexes are efficiently recognized by plasmacytoid dendritic cells (pDCs) and other antigen presenting cells (APC) via FcR-mediated uptake, and give rise to IFN-alpha production through TLR7/9 interactions in the endosomal compartment, a process inhibited by the binding of C1q to LAIR-1 (7). IFN-alpha has been reported to have many different adjuvant functions including up-regulation of the antigen presentation capacity of APC and increased production of class-switched antibodies by plasma cells, thus resulting in a vicious circle of increased autoantibody-self antigen interactions. Many novel therapeutic targets have been proposed, such as modulation of the LAIR-1/LAIR-2 axes (LAIR-2 being a soluble LAIR-1 inhibitor), RNase-mediated degradation of circulating RNA-containing complexes, and NETosis inhibition via PAD4 targeting. Several microRNAs (miRNAs) have been implicated in lupus pathogenesis by participating in the regulation of apoptosis, NETosis, antigen presentation, and production of IFN-alpha, and could theoretically be targeted via antigomirs. Further studies are needed to elucidate which of those may represent potential targets in human SLE.
