Abstract
Stimulation of P2X receptors by ATP in vascular smooth muscle cells (VSMCs) is proposed to mediate vascular tone. However, understanding of P2X receptor-mediated actions in human blood vessels is limited, and therefore, the current work investigates the role of P2X receptors in freshly isolated small human gastro-omental arteries (HGOAs). Expression of P2X1 and P2X4 receptor subunit messenger RNA (mRNA) and protein was identified in individual HGOA VSMCs using RT-PCR and immunofluorescent analysis and using Western blot in multi-cellular preparations. ATP of 10 μmol/l and αβ-meATP of 10 μmol/l, a selective P2X receptor agonist, evoked robust increases in [Ca2+]i in fluo-3-loaded HGOA VSMCs. Pre-incubation with 1 μmol/l NF279, a selective P2X receptor antagonist, reduced the amplitude of αβ-meATP-induced increase in [Ca2+]i by about 70 %. ATP of 10 μmol/l and αβ-meATP of 10 μmol/l produced similar contractile responses in segments of HGOA, and these contractions were greatly reduced by 2 μmol/l NF449, a selective P2X receptor inhibitor. These data suggest that VSMCs from HGOA express P2X1 and P2X4 receptor subunits with homomeric P2X1 receptors likely serving as the predominant target for extracellular ATP.
Electronic supplementary material
The online version of this article (doi:10.1007/s11302-014-9415-6) contains supplementary material, which is available to authorized users.
Keywords: Human, Gastro-omental arteries, ATP, P2X1 receptor, NF279
Introduction
The sympathetic nervous system increases vascular tone by releasing two major neurotransmitters, noradrenaline and ATP [4, 5]. It is now established that P2X receptors, ligand-gated Ca2+-permeable cation channels expressed in the plasma membrane of vascular smooth muscle cells (VSMC), are responsible for vasoconstrictor responses induced by neuronal released ATP. In muscular arteries, it is proposed that activation of P2X receptors leads to membrane depolarisation, followed by an increase in intracellular Ca2+ concentration ([Ca2+]i) and vasoconstriction, which evokes a rise in blood pressure [17, 24]. Therefore P2X receptors may represent an important therapeutic target for treatment of hypertension.
Since the proposal of purinergic neurotransmission by Burnstock in 1972 [3], seven different subunits for P2X receptors (P2X1-7) have been identified in mammalian tissues. P2X receptors subunits can combine to form functional homomeric and heteromeric ion channels which have distinctive pharmacological and biophysical properties [23, 33]. Immunoreactivity to P2X1, P2X2 and P2X4 receptor subunits has been reported in the mammalian vasculature, with homomeric P2X1 receptors likely to be the major contributor to ATP-mediated responses [28]. However, knowledge regarding the properties and contribution of P2X receptors in human blood vessels is limited. In this study, we investigated the expression, distribution, and function of P2X receptors in small human abdominal arteries, which are important vessels in controlling systemic vascular resistance and blood pressure. A preliminary account of some of this work has been previously reported in abstract form [20].
Materials and methods
Tissue retrieval and preparation
This investigation conforms to the principles in the Declaration of Helsinki and was approved by the Research Ethics Committee (09/H0803/103) for retrieval of human tissue. Abdominal adipose samples were obtained from 11 patients (four males and seven females, average age 47 ± 4 years) undergoing abdominal surgery. Consent was obtained from patients prior to surgery. Adipose tissue containing branches of human gastro-omental arteries (HGOAs) was dissected during surgery, and immediately transferred into cold (4 °C) physiological saline solution (PSS) following composition of (mmol/l): KCl 6, NaCl 120, MgCl2 1.2, CaCl2 2.0, d-glucose 10 and HEPES 10; pH adjusted to 7.4 with NaOH. Blood vessels were then dissected as described previously [10]. The arteries were cleaned of connective tissue (Supplementary Fig. 1), and their diameter was estimated at zero stretch under a dissection microscope using 0.1-mm micrometer slide. The resistance-sized [19] arteries with outer diameter less than 500 μm were used in the study. The arterial preparations were used in tension recording experiments and Western blot analysis (stored at −80 °C), or they were dispersed into single cells using enzymatic digestion [12] and then used for fluorescent [Ca2+]i imaging and immunofluorescence experiments. For reverse transcription polymerase chain reaction (RT-PCR) analysis, a glass pipette attached to a micromanipulator was used to collect individual freshly isolated VSMCs displaying the contractile phenotype (~200 cells for each analysis). The contractile properties of isolated myocytes were confirmed with application of high potassium (60 mmol/l) solution to the experimental chamber. The non-contractile cells displaying morphological properties of the phenotypically modulated vascular myocytes, such as filopodia, [10, 12, 13] were omitted during collection. The description of the cell collection procedure can be found in our previous work [10].
Fluorescent [Ca2+]i imaging
The agonist-induced changes in [Ca2+]i were monitored in HGOA VSMCs pre-loaded with Ca2+-sensitive indicator fluo-3 AM using the x-y mode of a Zeiss LSM 510 laser scanning confocal microscope (Oberkochen, Germany) as previously described [9].
Gene and protein expression analysis
Total RNA extraction, reverse transcription reaction and PCR were performed as in our previous experiments [10]. Thirty-five amplification cycles were performed for samples from whole arterial fragments, and 45 amplification cycles were performed for cDNA samples from individually collected cells. The details of primers used in RT-PCR analysis are shown in Table 1.
Table 1.
Primers used in RT-PCR analysis
| Gene name | Accession number | Amplicon location | Forward primer | Reverse primer |
|---|---|---|---|---|
| RPLP1 | NM_001003 | 289-462 | gtcaacattgggagcctcat | accaaagcccatgtcatcat |
| P2X1R | NM_002558.2 | 1334-1500 | tggagaacgggaccaactac | cagcagcaggtcacagagaa |
| P2X2R | NM_170682 | 555-734 | ccaatttctgggtacgatgg | acgataaagcccagcttgaa |
| P2X3R | NM_002559 | 897-1055 | tgtccccaggctacaacttc | ccacagagctgatgatggtg |
| P2X4R | NM_002560.2 | 974-1128 | gatcccttctgccccatatt | gagtacctgggcaagcagag |
| P2X5R | NM_002561.2 | 771-919 | tgtgagatctttgcctggtg | ttgacgtccatcacattgct |
| P2X6R | NM_005446.3 | 575-710 | aggcccagaacttcacactg | taggggctgaattgtggttc |
| P2X7R | NM_002562.5 | 947-1092 | ctgccgtcccaaatacagtt | aacggatcccgaagactttt |
For Western blot analysis, tissue samples were defrosted, homogenised in ice-cold RIPA Lysis Buffer System (Santa Cruz Biotechnology, Santa Cruz, USA) containing protease inhibitors, further disrupted by sonication on ice, and then centrifuged at 15,000 g for 20 min at 4 °C. The supernatant was collected and frozen at −80 °C. Protein content was quantified using the Bio-Rad DC Protein Assay method. Samples of supernatant were eluted with Laemmli sample buffer (dilution 1:1) and used in one-dimensional protein gel electrophoresis. One-dimensional protein gel electrophoresis was performed in 4–12 % Bis-Tris gels in a Novex mini gel system (Invitrogen, Paisley, UK). Proteins were transferred onto PVDF membranes using iBlot (Invitrogen, Paisley, UK) and then incubated with rabbit anti-P2X1 and rabbit anti-P2X4 primary antibodies at 1:1000 dilution overnight at 4 °C. Membranes were then washed and incubated with a donkey anti-rabbit horseradish-peroxidise-conjugated secondary antibody at 1:400 dilution (Thermo Fisher Scientific, Loughborough, UK), treated with electrochemiluminescence reagents (Pierce Biotechnology, Inc., Rockford, USA) for 1 min and then exposed to photographic films.
Fluorescent immunodetection of proteins using specific antibodies to P2X1 and P2X4 receptors at 1:300 dilution was performed according to protocols previously described [10]. Fluorescence was visualised using high resolution x-y mode of a Zeiss LSM 510 laser scanning confocal microscope.
Isometric tension recording
Arteries were cleaned of adherent tissue, and the endothelium was removed by passing an air bubble through the lumen of artery [35]. Artery segments of approximately 3 mm in length were mounted on a small wire myograph (Danish Myo Technology, Aarhus, Denmark) containing standard PSS bubbled with 95 % O2w/5 % CO2 and maintained at 37 °C. Arterial segments were allowed to equilibrate for 60 min; during this time, the segments were stretched gradually to a resting tension of 4 mN [18], and the bath solution was exchanged several times. Vascular function was tested with 60 mmol/l KCl following washout with PSS. The successful removal of the endothelium was confirmed in arteries pre-constricted with 10 μmol/l phenylephrine with application of 1 μmol/l acetylcholine for each preparation. No significant vasorelaxation was observed in samples used in this study. In each arterial ring, the agonists were applied twice with 20-min interval allowing the complete recovery of P2X receptors from desensitisation (please see “Results” section). The changes in tension were recorded using PowerLab and Chart software (ADInstruments, Oxford, UK).
Statistical analysis
All data shown is mean ± SEM calculated from n number of measurements. Statistical significance was calculated using Student’s t test for unpaired observations, with p < 0.05 considered significant.
Materials
All general chemicals including proteolytic enzymes were purchased from Sigma-Aldrich (Poole, UK). NF279 and NF449 were obtained from Tocris Bioscience (Bristol, UK). Molecular biology reagents and primers were purchased from Invitrogen (Paisley, UK), apart from RNeasy extraction kit which was purchased from Qiagen (Crawley, UK). Rabbit anti-P2X1, rabbit anti-P2X4 and rabbit anti-P2X7 antibodies were all from Abcam (Cambridge, UK). Donkey anti-rabbit MFP 488 secondary antibody was from Mobitec (Gottingen, Germany).
Results
P2X receptor gene and protein expression
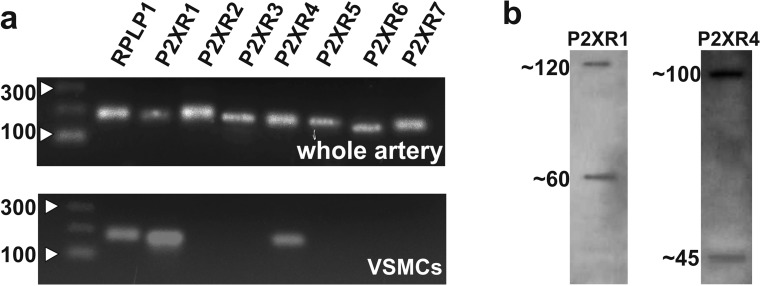
We initially investigated expression of P2X receptors in HGOA VSMCs using RT-PCR and Western blotting. In artery segments, messenger RNAs (mRNAs) for all seven subtypes of P2X receptor subunits were shown to be expressed (Fig. 1a, top). However, since these findings are from multi-cellular preparations, they do not show which P2X subtypes are present in VSMCs. Therefore, we carried out RT-PCR analysis using individually collected VSMCs, which showed that only P2X1 and P2X4 subunit mRNA were expressed in six preparations from five different patients (Fig. 1a, bottom). Immunoblotting with anti-P2X1 antibodies from HGOA preparations detected the presence of two proteins of ~60 and ~120 kDa (Fig. 1b, left). Human recombinant and native P2X1 receptor product with mass of ~60 kDa was reported in a number of publications [7, 15, 16, 22], and its glycosylated nature was confirmed [7], whereas 120-kDa product was confirmed as a dimer of the glycosylated P2X1 receptor subunit [15, 16, 22]. Immunoblotting with anti-P2X4 antibodies revealed products of ~43 and ~100 kDa (Fig. 1b, right). The ~43-kDa protein corresponds to the predicted molecular weight of monomeric P2X4 receptor subunit [32], whereas the ~100-kDa protein possibly represents a glycosylated monomeric P2X4 receptor subunit since the ~100-kDa product for P2X4 receptor was reported in human airway epithelial cells as a glycosylated form of the P2X4 receptor subunit [36]. Further studies, however, are required to confirm the nature of this product.
Fig. 1.
a RT-PCR analysis shows the expression of all P2X receptor subunit genes in whole HGOA (top panel), while the preparations from individually collected VSMCs show the presence of genes for only P2X1 and P2X4 receptor subunits (bottom panel) (analysis was performed in six samples from five patients). b Western blot analysis of the suspension of HGOA VSMCs shows the presence of monomeric and dimeric glycosylated by-products of P2X1 (60 and 120 kDA) and monomeric and glycosylated monomeric P2X4 (~45 and ~100 kDa) receptor subunits (n = 5)
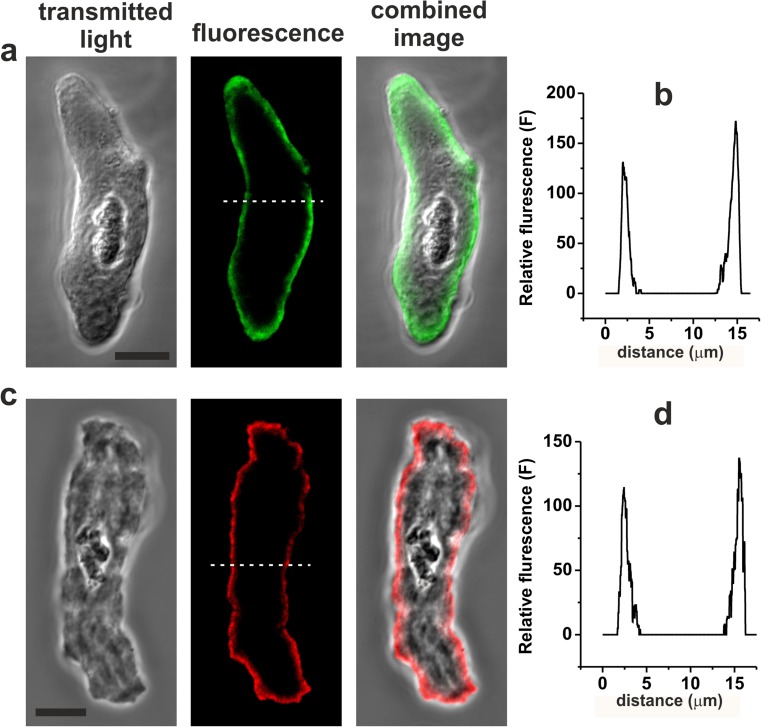
The cellular distributions of P2X1 and P2X4 receptor subunits in HGOA VSMCs were investigated using immunocytochemistry and single-cell confocal microscopy using five cell dispersals from five different patients. These experiments revealed that both P2X1 and P2X4 receptor subunit proteins were mainly observed at, or close to, the plasma membrane of VSMCs (Fig. 2). Our RT-PCR data indicated that P2X7 subunit mRNA is not expressed in HGOA VSMCs, although these subunits have been suggested to be expressed in human placenta VSMCs [34]. However, we found no detectable immunofluorescence for P2X7 subunits in HGOA VSMCs (data not shown). Taken together, these findings indicate that HGOA VSMCs express both P2X1 and P2X4 receptor subunits.
Fig. 2.
Immunofluorescent detection of P2X1 (a, b) and P2X4 (c, d) receptor subunits in HGOA VSMCs. Panels a, c show transmitted light, fluorescent and combined images of single VSMCs. Panels b, d show the graphs of intensity profile of the relative immunofluorescence plotted along the white dashed lines shown in the fluorescent images. The analysis shows that both proteins are localised to plasmalemmal region of the cells. Horizontal bars in transmitted light images correspond to 10 μm
Agonist-induced [Ca2+]i changes in HGOA VSMCs
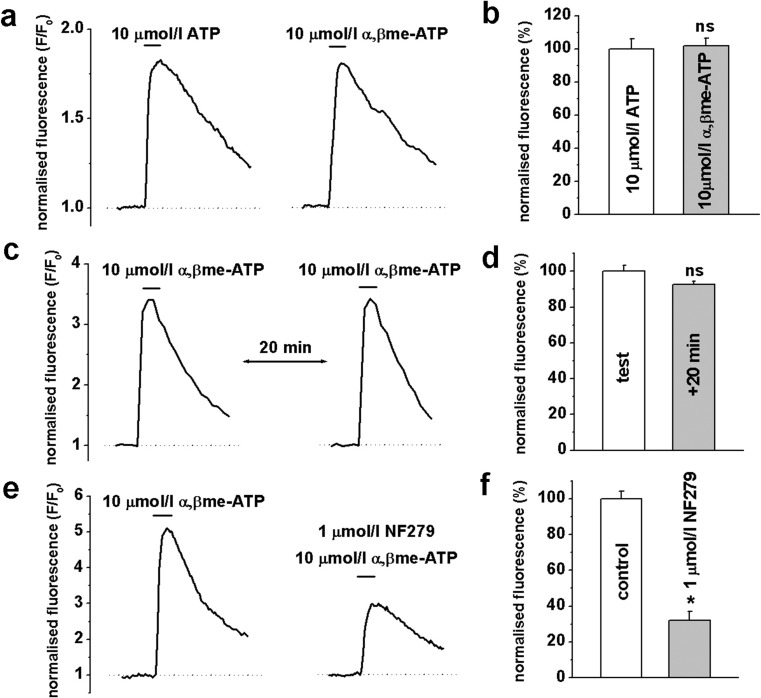
Increases in [Ca2+]i lead to activation of Ca2+/calmodulin-dependent myosin light chain kinase and initiation of contractile process, which is a pivotal step in the production of vascular tone [31]. Therefore, we investigated the effect of ATP and αβ-meATP, a potent selective P2X receptor agonist, on [Ca2+]i changes in fluo-3 pre-loaded HGOA VSMCs. Applications of 3-s pulses of 10 μmol/l ATP or 10 μmol/l αβ-meATP evoked robust increases in [Ca2+]i with similar kinetics and peak amplitudes (Fig. 3a). Mean normalised peak amplitudes of αβ-meATP-evoked changes in fluorescence were 101.7 ± 4.8 % compared to 100 ± 6.2 % for ATP-evoked responses (n = 6, p > 0.05) (Fig. 3b). As previously shown in animal studies [26], repetitive applications of 10 μmol/l αβ-meATP induced response desensitisation. In our experiments, 10 μmol/l αβ-meATP-mediated increases in [Ca2+]i showed near complete recovery following a 20-min interval between applications, with a mean normalised peak fluorescence amplitude of the second response being 92.6 ± 1.2 % compared to 100 ± 3.4 % for the first response (n = 6, p > 0.05) (Fig. 3c, d). To further confirm the involvement of P2X receptors, the effect of a selective P2X receptor blocker NF279 was studied on [Ca2+]i responses evoked by 10 μmol/l αβ-meATP. Pre-treatment of VSMCs with 1 μmol/l NF279 for 10 min significantly inhibited αβ-meATP-evoked increases in [Ca2+]i by about 70 % (Fig. 3e, f). These results provide strong evidence that P2X receptors mediate ATP-induced responses in HGOA VSMCs.
Fig. 3.
Agonist-induced changes in [Ca2+]i in HGOA VSMCs. Panel a shows representative traces of fluo-3 normalised fluorescence evoked by 3-s application of 10 μmol/l ATP and 10 μmol/l αβ-meATP. b Summarised data showed no significant difference in the effect of both agonists (n = 6, p > 0.05). c Repetitive application of 10 μmol/l αβ-meATP showed a nearly complete recovery of the agonist-induced [Ca2+]i response within 20-min interval (summary is shown in d; n = 6, p > 0.05). e Original traces of the normalised fluorescence showing that 1 μmol/l of potent P2X1 receptor blocker NF279 significantly decreased agonist-induced [Ca2+]i responses. f Summary of the mean amplitude of the normalised fluorescence in control and in the presence of the blocker (n = 6, p < 0.05, indicated by *; ns not significant
Agonist-induced contractile responses in multi-cellular preparations
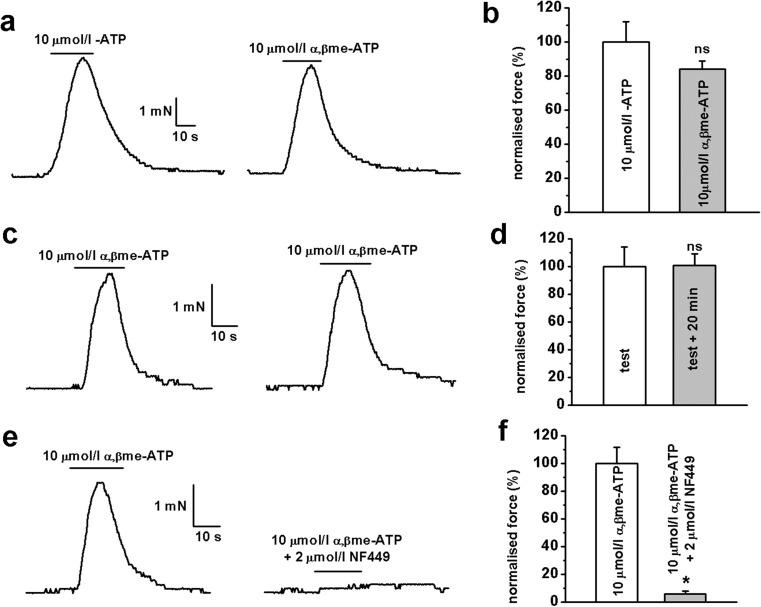
To examine the role of P2X receptors in controlling vascular tone, we investigated the pharmacological properties of ATP- and αβ-meATP-mediated vasoconstrictions using isometric tension recording. Application of 10 μmol/l ATP and 10 μmol/l αβ-meATP evoked similar contractile responses of artery segments, with a mean normalised peak amplitude of 100 ± 12 % for ATP (n = 6) and 84 ± 5 % for αβ-meATP (n = 10, p > 0.05) (Fig. 4a, b). To exclude possible involvement of P2Y receptors in ATP-mediated contractions, we used the selective P2X receptor agonist, αβ-meATP, in all subsequent experiments. Contractile responses evoked by repetitive applications of 10 μmol/l αβ-meATP showed complete recovery following a 20-min interval, with the mean normalised peak amplitude for the second response being 101 ± 8 % compared to 100 ± 14 % for the first response (n = 8, p > 0.05) (Fig. 4c, d). Finally, we studied the effect of the potent P2X receptor inhibitor NF449 on αβ-meATP-evoked contractile responses. Pre-treatment with 2 μmol/l NF449 for 10 min almost completely blocked αβ-meATP-evoked contractile responses with a mean normalised peak contractile amplitude of 6 ± 2 % in the presence of the antagonist compared to 100 ± 12 % in the absence of antagonist (n = 5, p < 0.05; Fig. 4e, f).
Fig. 4.
Agonist-induced contractile responses in HGOA preparations. a) Both 10 μmol/l ATP and 10 μmol/l αβ-meATP evoked similar contractile responses. b Summarised data showed no significant difference in the effect of both agonists (n = 6 for ATP and n = 10 for αβ-meATP, p > 0.05). c Repetitive applications of 10 μmol/l αβ-meATP revealed virtually complete recovery of the contractile responses after 20 min in the absence of the agonist. d Summarised data show no significant difference in averaged amplitude of normalised responses (n = 8, p > 0.05). e Ten-min pre-incubation of HGOA preparations with 1 μmol/l selective P2X receptor antagonist NF449 showed virtually complete inhibition of contractile responses evoked by 10 μmol/l αβ-meATP. Panel f shows summarised data (n = 5, p < 0.05, indicated by *); ns not significant
Discussion
It is now established that ligand-gated P2X Ca2+-permeable cation channels are widely expressed in the vasculature and play an important role in mediating the control of vascular tone by the sympathetic nervous system. The present study provides evidence that P2X1 and P2X4 receptor subunits are expressed in HGOA VSMCs and may have an important role in ATP-mediated responses in HGOA VSMCs.
mRNA expression analysis performed in multi-cellular preparations of HGOA showed the expression of genes for all seven P2X receptor subtypes, while individually collected VSMCs showed expression for only P2X1 and P2X4 receptor subunits. This indicates that P2X receptors other than P2X1 and P2X4 were expressed in other cells that constitute blood vessels such as neurons, endothelial cell and fibroblasts.
Our functional data also indicate that P2X1 and P2X4 subunits mediate P2X receptors present in HGOA VSMCs. ATP and the selective P2X receptor agonist αβ-meATP evoked similar increases in [Ca2+]i in fluo-3 pre-loaded VSMCs and similar contractile responses in segments of intact arteries. In addition, αβ-meATP-mediated changes in [Ca2+]i and contractions were inhibited by the selective P2X1 inhibitors NF279 and NF449, respectively. NF279 of 1 μmol/l used in the present work has been previously shown to produce near complete inhibition of recombinant homomeric P2X1 receptors [29] and native [11] P2X1 receptors. Moreover, 2 μmol/l NF449 used in this study would be expected to completely inhibit homomeric P2X1 and heteromeric P2X1/4 receptors but not homomeric P2X4 receptors [2, 11, 14]. It is therefore possible that apart from homomeric P2X1, homomeric P2X4 receptors or heteromeric P2X1/4 receptors could contribute ATP-mediated responses in HGOA VSMCs. However, homomeric P2X4 receptors are virtually insensitive to concentration of αβ-meATP used in our experiments [23], and expression of homomeric P2X4 receptors is known to be scarce due to their preferred heteromerisation with other P2X receptor subunits [21]. We therefore propose that homomeric P2X1 receptor plays a predominant role in P2X-mediated responses in HGOA VSMCs. Our data also allow us to hypothesise that the fraction of the purinergic response that was insensitive to 1 μmol/l NF279 could be mediated by heteromeric P2X1/4 receptors.
There is currently little knowledge regarding molecular and functional expression of P2X receptors in human vasculature. Immunoreactivity for P2X1 receptors and αβ-meATP-evoked contractile responses was reported in post-mortem human cerebral artery preparations [1]. The expression of transcripts for P2X1 and P2X7 receptor subunits was reported in human saphenous vein lysates [6]. The immunoreactivity for P2X1 receptors and corresponding functional responses were observed in human umbilical arteries [1]. Also, gene expression for P2X1, P2X4, P2X5, P2X6 and P2X7 receptors was identified in multi-cellular preparations from chorionic and umbilical arteries and veins [34]. Thus, the current knowledge is limited to larger-than-resistance-sized blood vessels or to studies in vessels lacking sympathetic innervations (placental vasculature). Therefore, we believe that the present work provides the first information on the role of P2X receptors in small human arteries.
P2X receptors in resistance blood vessels may serve as an important target for therapeutic intervention for pathological conditions associated with altered vascular tone such as hypertension, since P2X-mediated vasoconstriction may represent a significant vasoconstrictor drive resistant to current drug therapies. In fact, in some resistance blood vessels such as small arterioles, the response to sympathetic nerve stimulation is thought to be mediated solely by P2X receptors [8]. Furthermore, recent studies demonstrated that purinergic neurotransmission becomes predominant over adrenergic signalling in rat resistance blood vessels during elevated blood pressure [25, 30]. Interestingly, our preliminary studies in rat cerebral arteries showed that purinergic stimulation evoked potent contractions in arterial segments and strong [Ca2+]i responses in isolated VSMCs, while responses to noradrenaline were barely detectable [27].
In summary, our study demonstrates that out of seven P2X receptor subunits, only P2X1 and P2X4 are expressed in HGOA VSMCs and that functional contractile responses to ATP are mediated by P2X receptors in this tissue.
Electronic supplementary material
(DOC 458 kb)
Acknowledgments
This work was supported by the British Heart Foundation Intermediate Basic Science Research Fellowship to M.I.H. (FS/06/077) and BHF grant (PG/08/062/) to D.V.G. and M.I.H.
References
- 1.Bo X, Sexton A, Xiang Z, Nori SL, Burnstock G. Pharmacological and histochemical evidence for P2X receptors in human umbilical vessels. Eur J Pharmacol. 1998;353(1):59–65. doi: 10.1016/S0014-2999(98)00383-5. [DOI] [PubMed] [Google Scholar]
- 2.Braun K, Rettinger J, Ganso M, Kassack M, Hildebrandt C, Ullmann H, Nickel P, Schmalzing G, Lambrecht G. NF449: a subnanomolar potency antagonist at recombinant rat P2X1 receptors. Naunyn Schmiedebergs Arch Pharmacol. 2001;364(3):285–290. doi: 10.1007/s002100100463. [DOI] [PubMed] [Google Scholar]
- 3.Burnstock G. Purinergic nerves. Pharmacol Rev. 1972;24(3):509–581. [PubMed] [Google Scholar]
- 4.Burnstock G. Local mechanisms of blood flow control by perivascular nerves and endothelium. J Hypertens Suppl. 1990;8(7):S95–S106. [PubMed] [Google Scholar]
- 5.Burnstock G. Noradrenaline and ATP as cotransmitters in sympathetic nerves. Neurochem Int. 1990;17(2):357–368. doi: 10.1016/0197-0186(90)90158-P. [DOI] [PubMed] [Google Scholar]
- 6.Cario-Toumaniantz C, Loirand G, Ladoux A, Pacaud P. P2X7 receptor activation-induced contraction and lysis in human saphenous vein smooth muscle. Circ Res. 1998;83(2):196–203. doi: 10.1161/01.RES.83.2.196. [DOI] [PubMed] [Google Scholar]
- 7.Clifford EE, Parker K, Humphreys BD, Kertesy SB, Dubyak GR. The P2X1 receptor, an adenosine triphosphate-gated cation channel, is expressed in human platelets but not in human blood leukocytes. Blood. 1998;91(9):3172–3181. [PubMed] [Google Scholar]
- 8.Evans RJ, Surprenant A. Vasoconstriction of guinea-pig submucosal arterioles following sympathetic nerve stimulation is mediated by the release of ATP. Br J Pharmacol. 1992;106(2):242–249. doi: 10.1111/j.1476-5381.1992.tb14323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harhun M, Gordienko D, Kryshtal D, Pucovsky V, Bolton T. Role of intracellular stores in the regulation of rhythmical [Ca(2+)]i changes in interstitial cells of Cajal from rabbit portal vein. Cell Calcium. 2006;40(3):287–298. doi: 10.1016/j.ceca.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 10.Harhun MI, Huggins CL, Ratnasingham K, Raje D, Moss RF, Szewczyk K, Vasilikostas G, Greenwood IA, Khong TK, Wan A, Reddy M. Resident phenotypically modulated vascular smooth muscle cells in healthy human arteries. J Cell Mol Med. 2012;16(11):2802–2812. doi: 10.1111/j.1582-4934.2012.01609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harhun MI, Povstyan OV, Gordienko DV. Purinoreceptor-mediated current in myocytes from renal resistance arteries. Br J Pharmacol. 2010;160(4):987–997. doi: 10.1111/j.1476-5381.2010.00714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harhun MI, Szewczyk K, Laux H, Prestwich SA, Gordienko DV, Moss RF, Bolton TB. Interstitial cells from rat middle cerebral artery belong to smooth muscle cell type. J Cell Mol Med. 2009;13(11–12):4532–4539. doi: 10.1111/j.1582-4934.2008.00567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huggins CL, Povstyan OV, Harhun MI. Characterization of transcriptional and posttranscriptional properties of native and cultured phenotypically modulated vascular smooth muscle cells. Cell Tissue Res. 2013;352(2):265–275. doi: 10.1007/s00441-012-1541-2. [DOI] [PubMed] [Google Scholar]
- 14.Hulsmann M, Nickel P, Kassack M, Schmalzing G, Lambrecht G, Markwardt F. NF449, a novel picomolar potency antagonist at human P2X1 receptors. Eur J Pharmacol. 2003;470(1–2):1–7. doi: 10.1016/S0014-2999(03)01761-8. [DOI] [PubMed] [Google Scholar]
- 15.Jiang L, Bardini M, Keogh A, dos Remedios CG, Burnstock G. P2X1 receptors are closely associated with connexin 43 in human ventricular myocardium. Int J Cardiol. 2005;98(2):291–297. doi: 10.1016/j.ijcard.2003.11.036. [DOI] [PubMed] [Google Scholar]
- 16.Lecut C, Frederix K, Johnson DM, Deroanne C, Thiry M, Faccinetto C, Maree R, Evans RJ, Volders PG, Bours V, Oury C. P2X1 ion channels promote neutrophil chemotaxis through Rho kinase activation. J Immunol. 2009;183(4):2801–2809. doi: 10.4049/jimmunol.0804007. [DOI] [PubMed] [Google Scholar]
- 17.Li L, Wu Y, Deng M, Wu G, Ren L. P2X1 receptor-mediated pressor responses in the anesthetized mouse. Acta Pharm Sin B. 2012;2(5):459–463. doi: 10.1016/j.apsb.2012.04.005. [DOI] [Google Scholar]
- 18.Malmsjo M, Hou MF, Pendergast WF, Erlinge DF, Edvinsson L. Potent P2Y6 receptor mediated contractions in human cerebral arteries. BMC Pharmacol. 2003;3:4. doi: 10.1186/1471-2210-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mulvany MJ, Aalkjaer C. Structure and function of small arteries. Physiol Rev. 1990;70(4):921–961. doi: 10.1152/physrev.1990.70.4.921. [DOI] [PubMed] [Google Scholar]
- 20.Nichols CM, Povstyan OV, Khan O, Vasilikostas G, Khong TK, Wan A, Reddy M, Harhun MI (2013) Molecular identification of P2X receptors in human arteries. Proc 37th IUPS, PCA407
- 21.Nicke A, Kerschensteiner D, Soto F. Biochemical and functional evidence for heteromeric assembly of P2X1 and P2X4 subunits. J Neurochem. 2005;92(4):925–933. doi: 10.1111/j.1471-4159.2004.02939.x. [DOI] [PubMed] [Google Scholar]
- 22.Nicke A, Rettinger J, Schmalzing G. Monomeric and dimeric byproducts are the principal functional elements of higher order P2X1 concatamers. Mol Pharmacol. 2003;63(1):243–252. doi: 10.1124/mol.63.1.243. [DOI] [PubMed] [Google Scholar]
- 23.North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82(4):1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- 24.Osmond DA, Inscho EW. P2X(1) receptor blockade inhibits whole kidney autoregulation of renal blood flow in vivo. Am J Physiol Renal Physiol. 2010;298(6):F1360–F1368. doi: 10.1152/ajprenal.00016.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pakdeechote P, Rummery NM, Ralevic V, Dunn WR. Raised tone reveals purinergic-mediated responses to sympathetic nerve stimulation in the rat perfused mesenteric vascular bed. Eur J Pharmacol. 2007;563(1–3):180–186. doi: 10.1016/j.ejphar.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 26.Povstyan OV, Harhun MI, Gordienko DV. Ca(2+) entry following P2X receptor activation induces IP(3) receptor-mediated Ca(2+) release in myocytes from small renal arteries. Br J Pharmacol. 2011;162(7):1618–1638. doi: 10.1111/j.1476-5381.2010.01169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Povstyan OV, Nichols CM, Harhun MI (2013) Identification of functional P2X receptors in rat middle cerebral arteries. Proc 37th IUPS PCA395-
- 28.Ralevic V. P2X receptors in the cardiovascular system. WIREs Membr Transp Signal. 2012;1(5):663–674. doi: 10.1002/wmts.58. [DOI] [Google Scholar]
- 29.Rettinger J, Schmalzing G, Damer S, Muller G, Nickel P, Lambrecht G. The suramin analogue NF279 is a novel and potent antagonist selective for the P2X(1) receptor. Neuropharmacology. 2000;39(11):2044–2053. doi: 10.1016/S0028-3908(00)00022-8. [DOI] [PubMed] [Google Scholar]
- 30.Rummery NM, Brock JA, Pakdeechote P, Ralevic V, Dunn WR. ATP is the predominant sympathetic neurotransmitter in rat mesenteric arteries at high pressure. J Physiol. 2007;582(Pt 2):745–754. doi: 10.1113/jphysiol.2007.134825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Somlyo AP. Excitation-contraction coupling and the ultrastructure of smooth muscle. Circ Res. 1985;57(4):497–507. doi: 10.1161/01.RES.57.4.497. [DOI] [PubMed] [Google Scholar]
- 32.Soto F, Garcia-Guzman M, Gomez-Hernandez JM, Hollmann M, Karschin C, Stuhmer W. P2X4: an ATP-activated ionotropic receptor cloned from rat brain. Proc Natl Acad Sci U S A. 1996;93(8):3684–3688. doi: 10.1073/pnas.93.8.3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Torres GE, Egan TM, Voigt MM. Hetero-oligomeric assembly of P2X receptor subunits. Specificities exist with regard to possible partners. J Biol Chem. 1999;274(10):6653–6659. doi: 10.1074/jbc.274.10.6653. [DOI] [PubMed] [Google Scholar]
- 34.Valdecantos P, Briones R, Moya P, Germain A, Huidobro-Toro JP. Pharmacological identification of P2X1, P2X4 and P2X7 nucleotide receptors in the smooth muscles of human umbilical cord and chorionic blood vessels. Placenta. 2003;24(1):17–26. doi: 10.1053/plac.2002.0862. [DOI] [PubMed] [Google Scholar]
- 35.Wallis SJ, Firth J, Dunn WR. Pressure-induced myogenic responses in human isolated cerebral resistance arteries. Stroke. 1996;27(12):2287–2290. doi: 10.1161/01.STR.27.12.2287. [DOI] [PubMed] [Google Scholar]
- 36.Zsembery A, Boyce AT, Liang L, Peti-Peterdi J, Bell PD, Schwiebert EM. Sustained calcium entry through P2X nucleotide receptor channels in human airway epithelial cells. J Biol Chem. 2003;278(15):13398–13408. doi: 10.1074/jbc.M212277200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 458 kb)