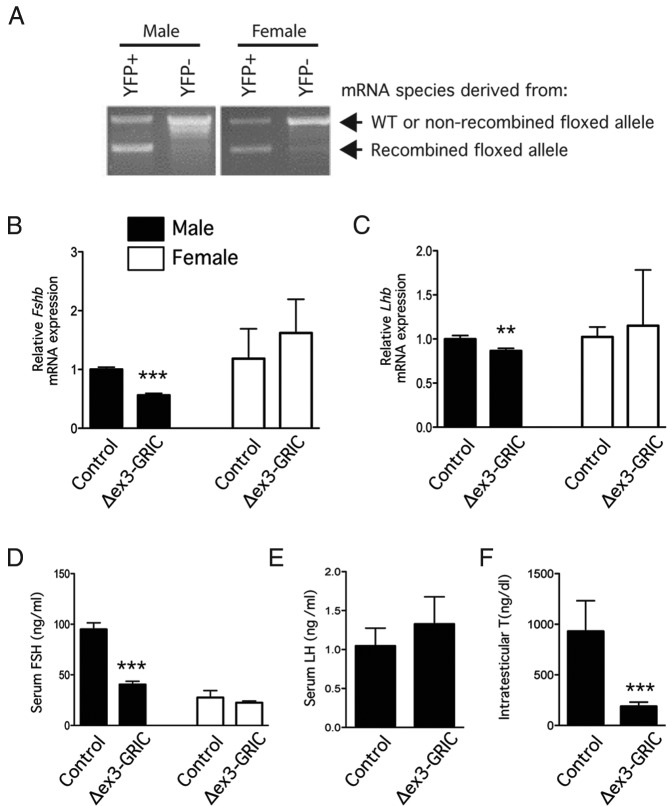
Figure 1. A male specific FSH synthesis defect occurs in the Δex3-GRIC model.
A, Recombination efficiency in Δex3-GRIC gonadotropes. Pituitary cells from male (left) and female (right) Δex3-GRIC;YFP mice were enzymatically dispersed and fluorescence-activated cell sorting sorted into YFP+ (ie, gonadotropes) and YFP− (ie, nongonadotropes) fractions. RT-PCR analysis of Ctnnb1 mRNA expression was then done using a primer pair that flanks the mRNA sequences encoded by the third exon and that, therefore, generates a smaller PCR product (lower band) after Cre-mediated recombination. As expected, YFP+ Δex3-GRIC;YFP pituitary cells produced 2 mRNA species (ie, derived from the wild-type and the Cre-recombined floxed alleles). PCR products were separated by electrophoresis on 1% agarose gels containing ethidium bromide and photographed under UV illumination. B and C, Pituitary gonadotropin subunit gene expression in adult Δex3-GRIC mice. Six-week-old female (n = 5–7 per genotype) and 8- to 12-week-old male (n = 18–20 per genotype) mice were killed and pituitary glands harvested for measurement of Fshb and Lhb expression by real-time RT-PCR. Serum was also collected for FSH (D) and LH (males only) (E) measurement. Intratesticular testosterone levels were measured from testis homogenates prepared from the same animals (F). Analyses shown in B–D were also done using 6-month-old female Δex3-GRIC mice, yielding similar results (data not shown). RT-PCR analyses were done separately on samples from male and female mice; relative expression, therefore, cannot be compared between genders. Columns, means; error bars, SEM. Significant difference from control is indicated with asterisks (**, P < .01; ***, P < .001, unpaired t test).