Fig. 4.
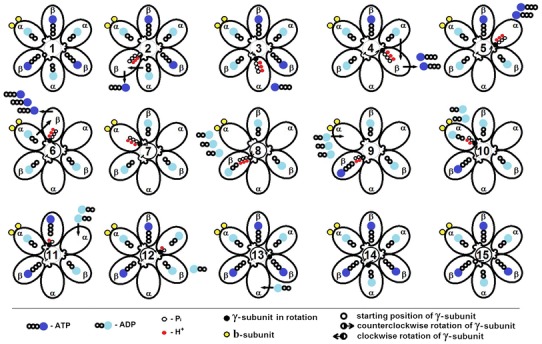
A rotary mechanism of the ATP synthesis, the release of ATP, and the loading of ADP. Arrows indicate the ATP release from the active site of β-subunit and the loading of ADP to the active site of α-subunit; the transition of ADP from the active center of α-subunit to active site of β-subunit. The arrows in the center of the figure show the rotation of the γ-subunit in steps of 30° and 90° counterclockwise direction and clockwise direction. 1 γ-subunit is in the starting position near the β-subunit. ADP and ATP are tightly bound in the α-subunits and β-subunits, respectively; 2 C-terminal of γ-subunit connects three protons and three phosphate ions during the energization process; the active center is opened and ATP molecule is released due to electrostatic repulsion from the active center and moves to the top of the α 3 β 3-hexamer; 3 γ-subunit starts to rotate counterclockwise direction and contacts the alpha subunit; and ADP moves from alpha subunit to the beta subunit; 4–8 γ-subunit continues to rotate counterclockwise direction and stops at the beta subunit, making 360°; at this time, all the molecules of ATP are in the upper part of the α 3 β 3-hexamer, and all ADP molecules are passed on to the beta subunits from the alpha subunits; and F1 is attracted to the membrane; 8, 9 three molecules of ATP in upper part of α 3 β 3-hexamer are exchanged with three molecules of ADP from the matrix in the presence of sodium ions during full energization of the system; 9 after full energization, the first molecule of ATP is synthesized in the β-subunit at the starting position of γ-subunit; 10 γ-subunit starts to rotate clockwise direction and contacts with the α-subunit. ADP molecule moves from the upper part of the α 3 β 3-hexamer into α-subunit; 11–15 γ-subunit continues to rotate clockwise direction and stops at the β-subunit, making 360°; at this time, three ATP molecules are synthesized and remain tightly bound on the β-subunits; at the same time, ADP molecules are bound on the alpha subunits; and F1 is spaced from the membrane during complete deenergization
